方忠祥副教授、谭洪卓研究员主持“澳大利亚农业、食品及其科教发展”特约专栏文章之五
贮藏和加工方法对可食用花卉香气影响的研究进展(中英文)
梁子健,杨婧雯,方忠祥✉
(墨尔本大学 理学部 农业、食品和生态系统科学学院,澳大利亚 维多利亚州,墨尔本 3010)
摘 要: 可食用花卉是植物的花朵部分,可食用但储藏寿命较短;由于其种间相似性,香气特性在商业品种中起着重要的质量指标作用。具有特征性香气的可食用花卉的不同部分已被广泛应用于食品中,如新鲜的玫瑰花瓣、芙蓉花萼、藏红花的干雌蕊、接骨木花糖浆和薰衣草精油。萜烯、萜类化合物和酚类化合物是常见的挥发性化合物,它们的功能性基团是香气特性的主要原因,包括醇类、醛类、酮类和酯类。在新鲜的可食用花卉中,大多数醇类和酯类赋予花香和甜香,大多数烯烃具有木质香气特点,而大多数醛类代表着绿色和柑橘香味。冷藏(0~4 ℃)和气调包装都可以保留可食用花卉中的大多数挥发性化合物,而总挥发性成分可以通过各种干燥技术来增强。与冷冻干燥相比,热风干燥技术可以生成更多的挥发性化合物比如醛类。萃取是一种多功能性的技术,可从可食用花卉中分离出最多的挥发性化合物。热水冲泡和水-蒸气蒸馏可以生成更多的醇类、醛类和酮类。为了保持可食用花卉中原有的挥发性成分,近年来人们已经开发出新的萃取技术,如顶空收集和冷凝处理,并在食品加工中应用。本综述为食品工业中如何在保持香气特性的条件下应用可食用花卉提供了参考。
关键词: 可食用花卉; 香气; 挥发性化合物; 储存; 干燥; 萃取
中图分类号: TS201.6
文献标识码: A
文章编号: 1007-7561(2025)01-0039-32
网络首发时间: 2024-12-09 13:07:45
网络首发地址: https://link.cnki.net/urlid/11.3863.TS.20241206.1640.002
DOI: 10.16210/j.cnki.1007-7561.2025.01.005
梁子健,杨婧雯,方忠祥. 贮藏和加工方法对可食用花卉香气影响的研究进展(中英文)[J]. 粮油食品科技,2025,33(1):39-70.
LIANG Z J, YANG J W, FANG Z X. Effect of storage and processing methods on the Aroma of edible flowers: a review( Chinese and English versions)[J]. Science and Technology of Cereals, Oils and Foods, 2025, 33(1):39-70.
收稿日期:2024-06-28;修回日期:2024-09-09;录用日期:2024-09-10
第一作者:梁子健,男,1994 年出生,博士,研究方向为葡萄酒化学、食品化学、食品加工与微生物学、细胞生物学,E-mail: zijian.liang1@unimelb.edu.au
通信作者:方忠祥,男,1970年出生,博士,副教授,研究方向为食品科学与工程,E-mail:zhongxiang.fang@unimelb.edu.au
英译中:梁子健、方忠祥(墨尔本大学 理学部 农业食品与生态系统科学学院)
本专栏背景及通信作者介绍详见PC18-21,本文英文原文详见P57-70。
Effect of Storage and Processing Methods on the Aroma of Edible Flowers:A Review (Chinese and English versions)
LIANG Zi-jian, YANG Jing-wen, FANG Zhong-xiang✉
(School of Agriculture, Food and Ecosystem Sciences, Faculty of Sciences, The University of Melbourne, Parkville, VIC 3010, Australia)
Abstract: Edible flowers are the floral parts of plants that are safe for consumption but have a short shelf life. Due to the interspecific similarity of edible flowers, their aroma properties serve as a vital quality indicator in most commercial flower species. Different parts of edible flowers with characteristic fragrances are widely applied in food products, such as fresh rose petals, hibiscus calyces, dried stigmas of crocus sativus, elderflower syrup, and lavender oil. Terpenes, terpenoids, and phenolics are the common volatile compounds, where the functional groups mainly contribute to the aroma characteristics, including alcohols, aldehydes, ketones, and esters. In fresh edible flowers, most alcohols and esters give a floral and sweet odor, most alkenes are characteristic of a woody aroma,while most aldehydes represent green and citrus scents. Both cold storage (0~4 °C) and modified atmosphere packaging could retain most of the volatiles in edible flowers. Additionally, the total volatile content of edible flowers could be enhanced using various drying technologies. Compared with freeze-drying, hot air drying technologies could generate more volatiles, especially aldehydes. Extraction is a versatile technology to isolate the highest amount of volatile compounds from edible flowers. More alcohols, aldehydes, and ketones are generated by hot water brewing and water-steam distillation. To maintain the original volatile profile, novel extraction technologies such as large-scale headspace collection and condensation processing have been developed and used in recent years. This review provides a reference for the application of edible flowers in the food industry without compromising the odor quality.
Key words: edible flowers; aroma; volatiles; storage; drying; extraction
花朵自古以来一直是一些国家烹饪食物的一部分,而可食用花卉近年来在全球范围内变得越来越受欢迎。可食用花卉不仅具有独特的风味和外观,还对人类健康有益。可食用花卉的特性使消费者不仅可以将其用作装饰,还可以用来制作主食,如花瓣蛋糕和烩饭[1]。为了满足人们对美食不断增长的需求,全球范围内涌现了越来越多的可食用花卉公司,这些公司采用了不同的储存和加工技术来贮存可食用花卉[2]。Zhao 等[3]和Fernandes等[4]综述了包括干燥技术和萃取技术在内的花卉加工技术。很多研究对可食用花卉的质量参数进行了探究,包括色素、脂质、抗氧化和抗菌性能等[5-7]。挥发性化合物在可食用花卉的质量方面起着关键作用,它们贡献了不同花卉的独特香气[2]。随着气相色谱(GC)-嗅觉分析技术的进步,可食用花卉的香气特性可以被高效地识别,因此储存和加工对可食用花卉挥发性化合物的影响能够被准确地分析。然而,目前关于可食用花卉挥发性化合物的文献综述尚不存在,本文旨在填补这一空白,为未来可食用花卉的食品工业应用提供参考。
1 可食用花卉的香气
1.1 可食用花卉的香气特性
香气是可食用花卉尤其是香气浓郁的花朵的主要特征之一,每个品种的花卉都具有独特的香气[2]。对于同一品种的可食用花卉,它们的香气特性主要源自于相似的挥发性化合物。此外,同一品种的相似部位,如花瓣、花萼、花蕾和花粉,展现出更高水平的香气强度。然而,新鲜可食用花卉一旦凋谢,其香气强度会迅速下降[2]。除了基因表达之外,香气特性还受到各种非挥发性化学成分(例如有机酸、糖类、酚类化合物)的影响[4]。在可食用花卉的加工过程中尽量减少挥发性化合物的损失是食品制造商为确保高质量产品而需要考虑的重要因素。表1列举了一些含有大量挥发性化合物的可食用花卉及其具有浓郁香气的商业产品。
表1 商品可食用花卉的通用名称、品种、产地、功效、食用部位及用途
Table 1 Common names, species, origins, potentia health benefits, edible parts and applications of selective commercial edible flowers
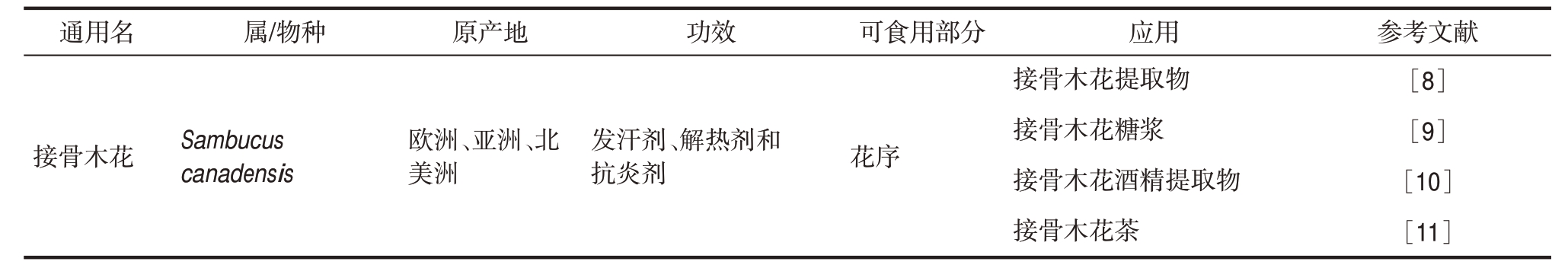
通用名 属/物种 原产地 功效 可食用部分 应用 参考文献接骨木花 Sambucus canadensis欧洲、亚洲、北美洲发汗剂、解热剂和抗炎剂 花序接骨木花提取物接骨木花糖浆接骨木花酒精提取物接骨木花茶[8][9][10][11]
续表1

通用名 属/物种 原产地 功效 可食用部分 应用 参考文献茉莉花Jasminum sambac 印度、阿拉伯抗关节炎花瓣[12][13][14][15][16][17][18][19]薰衣草Lavandula spica 地中海对抗食源性病原体花序洛神花或芙蓉花Hibiscus sabdariffa 印度、东南亚抗氧化剂、抗肿瘤剂、抗糖尿病剂和抗血脂剂花萼属玫瑰Rosa spp.东北亚缓解高血压花瓣藏红花Crocus sativus 美索不达米亚抗癌、抗过敏、抗衰老、抗肥胖干燥的柱头桂花Osmanthus fragrans中国花冠菊花Chrysanthemum morifolium 东亚、东北欧解热、解毒、活血花序接骨木花起泡酒波兰酒精饮料生吃茉莉花混凝土茉莉挥发性缩合物茉莉绿茶生吃配菜和甜点,如蛋奶冻和冰淇淋Hamsi kaygana(一种含有凤尾鱼的食物)薰衣草提取物薰衣草香精成分黑米和薰衣草精油干洛神花花萼凝胶食品、软饮料和烘焙产品,如披萨和蛋糕芙蓉酒洛神花巧克力蛋糕贝宁洛神花输液密相二氧化碳加工木槿饮料芙蓉水浸剂墨西哥芙蓉花萼热饮芙蓉果冻和果酱风味酸奶芙蓉发酵乳木槿花拼配茶芙蓉发酵产品生吃玫瑰花瓣片玫瑰悬浮汁新型红外线冻干玫瑰酸奶玫瑰蛋糕玫瑰果冻玫瑰饮料玫瑰花茶茶香玫瑰玫瑰醋意大利米兰烩饭、法国马赛鱼汤和西班牙海鲜饭藏红花冰淇淋芝士蛋糕和白巧克力汤配藏红花伊朗藏红花提取物生吃桂花蜜酒桂花与外源酶菊花茶[20][21][22][23][24][25][26-27][28][29][30][31-32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][3][48][49][50][51][16][52][53][54-55]
1.2 可食用花卉的挥发性物质
到目前为止,具有浓郁香气的各种可食用花卉已被鉴定出了数千种挥发性化合物,但其中只有少数是对整体香气感知产生影响的气味活性化合物[29,56]。这些挥发性物质的类型和浓度在不同可食用花卉品种之间存在差异,而萜类化合物是大多数可食用花卉中的主要挥发性化合物[4,57]。可食用花卉中大多数挥发性物质的分子结构具有含氧官能团,这些官能团可用于分类不同可食用花卉的特征香气[57]。对于表1中的可食用花卉,根据功能基团分类的气味活性化合物主要是醇类和醛类,其次是烯烃和酯类(表2)。大多数可食用花卉中的醇类和酯类挥发物呈现出花香,而烯烃则具有木质和蘑菇的气味。大多数醛类的气味特征是绿色和柑橘。除了气味特征,在加工过程中还应考虑可食用花卉挥发性物质的气味阈值和潜在安全风险(表3)。
表2新鲜可食用花和藏红花(干藏红花)的主要挥发性成分、气味描述及检测方法
Table 2 Main volatile compounds, odour description and detection methods of selective fresh edible flowers and saffron (dried Crocus sativus)
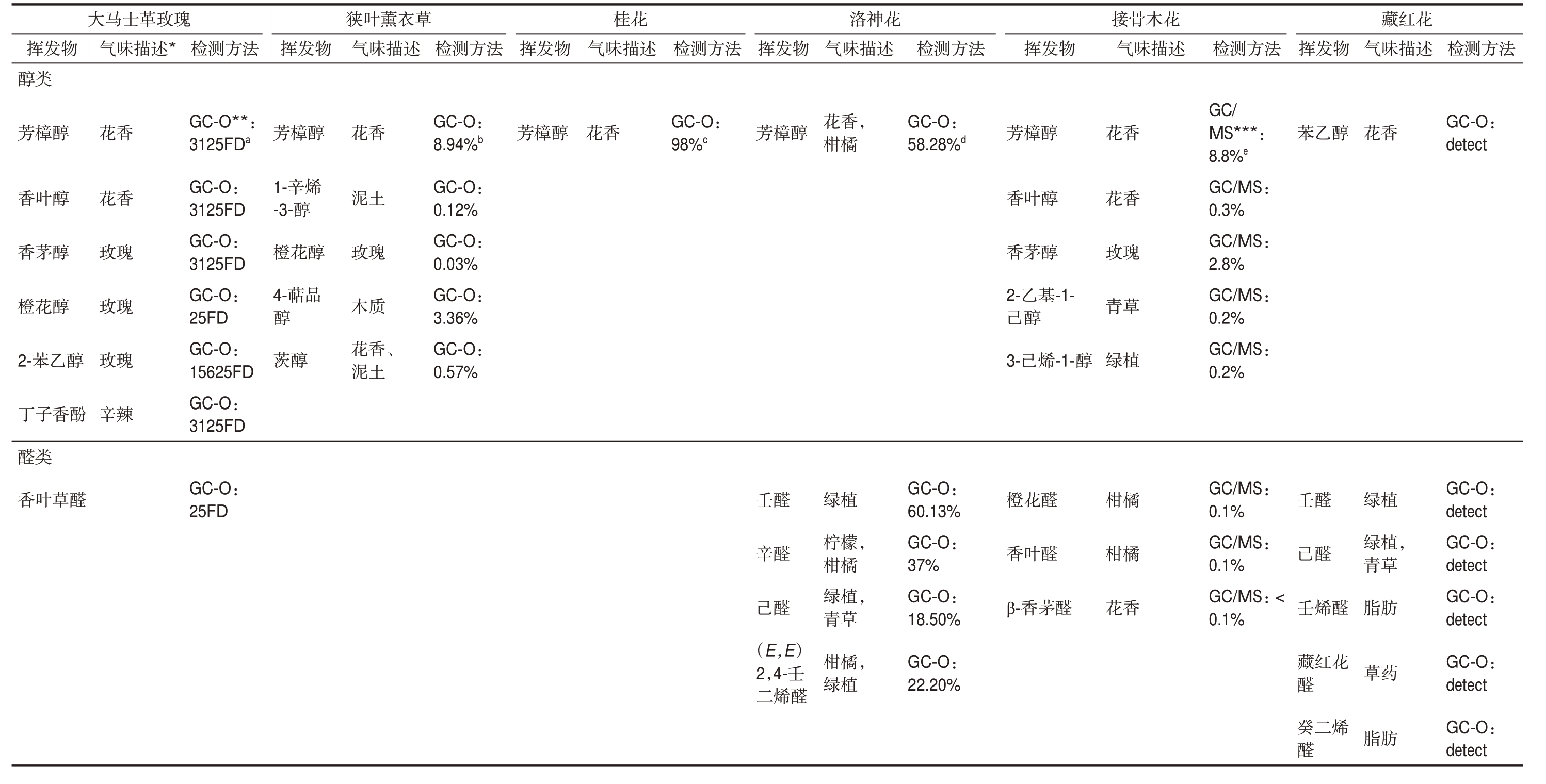
法方测检GC-O: detect GC-O: detect GC-O: detect GC-O: detect GC-O: detect GC-O: detect,狭叶薰衣草 桂花 洛神花 接骨木花 藏红花述描味香植气植草肪药肪花绿脂草绿青脂物发醇醛花烯乙挥醛醛烯红二壬苯己壬藏醛 癸醛法方检测GC/MS***: 8.8%e GC/MS: 0.3%GC/MS: 2.8%GC/MS: 0.2%GC/MS: 0.2%GC/MS: 0.1%GC/MS: 0.1%GC/MS: < 0.1%述香气描味瑰草植橘橘香青花花柑玫柑绿花物-1--1-醇醛醇发醇醇基醛烯挥醛茅茅叶樟花叶香香2-乙醇芳己3-己橙香β-香法方测检GC-O: 58.28%d GC-O: 60.13%GC-O: 37%GC-O: 18.50%GC-O: 22.20%描味,,,述,气香橘植檬橘植草橘植柠柑绿青花柑柑绿绿物醇发挥樟醛醛醛烯芳壬己二辛(E,E)2,4-壬醛法测检方GC-O: 98%c述描味气香花发醇樟物芳挥方香法测检GC-O: 8.94%b GC-O: 0.12%GC-O: 0.03%GC-O: 3.36%GC-O: 0.57%述描味、气香土瑰质香土花泥玫木花泥物发醇醇品挥樟花醇芳1-辛烯-3-醇橙4-萜醇 茨法方测检瑰*GC-O**: 3125FDa GC-O: 3125FD GC-O: 3125FD GC-O: 25FD GC-O: 15625FD GC-O: 3125FD GC-O: 25FD玫述革描士味马气香香瑰瑰瑰辣大花花玫玫玫辛物醇酚醛发醇醇醇醇乙香草挥类樟叶茅花子类叶醇芳香香橙2-苯丁醛香
续表2

注: *=气味描述来自文献; **=GC-O: 气相色谱-嗅觉分析; ***=GC-MS: 气相色谱-质谱分析; a=通过GC-O检测挥发物,并通过香气提取物稀释分析来分析风味稀释(FD)因素; b=GC-O检测到的挥发物以及基于顶空固相微萃取(HS-SPME)的值; c=通过GC-O检测挥发物,并使用Dravnieks公式计算修正频率(MF,%); d=通过GC-O检测挥发物并通过面积归一化方法鉴定归一化峰面积; e=挥发物通过气相色谱-质谱法(GC-MS)检测,值为GC-MS面积。
Note: *=Odor descriptions were obtained from literatures; **=GC-O: Gas chromatography-olfactometry analysis; ***=GC-MS: Gas chromatography-mass spectrometry analysis; a=volatiles are detected by GCOand the flavour dilution( FD) factors are analysed by aroma extract dilution analysis; b=volatiles are detected by GC-O and the value based on headspace solid-phase microextraction( HS-SPME); c=volatiles are detected by GC-O and modified frequency( MF,%) are calculated using the Dravnieks formula; d=volatiles are detected by GC-O and the normalized peak area are identified by area normalization method; e=volatiles are detected by Gas chromatography-mass spectrometry( GC-MS) and the value is GC-MS area.
法方测检GC-O: detect GC-O: detect GC-O: detect GC-O: detect GC-O: detect花述红描藏味气菇油质味士蘑黄木酸芝物发尔酸挥1-辛烯-3-酮2,3-丁酮佛酸戊二异酮醋异[61]法方测检GC/MS: < 0.1%GC/MS: < 0.1%GC/MS: 23.6%GC/MS: 0.1%GC/MS: 28.2%花述木描植骨味,绿接气荷瑰薄玫物醇物发樟化挥酮醚2-壬喃瑰(Z)-芳氧烯吡玫勒(E)-β-罗[60]法方测检GC-O: 18.50%GC-O: 50.88%花述神描洛味,气菇油香蘑黄花物发酮基挥3-辛叶酮香丙[30]法方测检GC-O: 17%GC-O: 17%GC-O: 82%GC-O: 54%GC-O: 46%GC-O: 65%述花描桂味、气质香质香植橘药木花木花绿柑草物发罗罗芳烯烯挥β-紫酮酸酯-3-己丁基酯檬勒兰α-紫酮兰乙樟顺烯酸柠罗[59]法方测检GC-O: 56.94%GC-O: 0.11%GC-O: 0.12%GC-O: 0.24%草衣述薰描叶味、草、、狭气香衣香瑰香瑰荷花薰花玫花玫薄物发芳乙叶乙叶精挥酸酯式香式香油乙樟顺酸酯反酸酯 桉[19]法方测GC-O: 3125FD检瑰*玫述革描士味马气瑰大玫献物醚文发类类他瑰考挥酮酯其玫参[58]
表3 可食用花卉中的主要挥发性化合物及其生物合成途径、潜在健康益处、气味阈值和安全问题
Table 3 Main volatile compounds in edible flowers and its biosynthesis pathway, potential health benefits,odour threshold and safety concern

注: *JECFA: 粮农组织/世界卫生组织食品添加剂联合专家委员会; *ADI: 可接受的每日摄入量; *N/A=数据不可用。
Note: *JECFA: Joint FAO/WHO Expert Committee on Food Additives; *ADI: Acceptable daily intake; *N/A=Data not available.
挥发物芳樟醇生物合成源自二磷酸香叶基[62]Odour threshold 3.2纳克/升空气[62]安全规定(JECFA*)ADI*: 0~0.5 mg/kg可食用花卉 [60]番红花、小苍兰、藿香茴香、薰衣草香叶醇源自二磷酸香叶基[64]2.8纳克/升空气[32]N/A*蔷薇、鸡蛋花、金盏花和忍冬香茅醇苯乙醇食品标签上的香料过敏原N/A丁子香酚ADI: 2.5 mg/kg马鞭草、枳壳、花椒和天竺葵玫瑰、石竹、风信子、依兰、天竺葵和黄玉兰蔷薇、罗勒和矮牵牛己醛N/A 木槿、蔷薇、矮牵牛藏红花醛N/A β-紫罗兰酮ADI: 0~0.1 mg/kg香叶基丙酮香叶醇还原酶反应[67]源自 L-苯丙氨酸[69]MEP途径[71]不饱和脂肪酸氧化[74]类胡萝卜素氧化[77-78]β-胡萝卜素裂解[80]八氢番茄红素裂解[83]N/A乙酸香叶酯ADI: 0~0.5 mg/kg乙酸芳樟酯乙酰转移酶(AAT)与醇的酶活性[85]ADI: 0~0.5 mg/kg乙酸香茅酯健康益处对抗病原真菌[63]抗肿瘤[65]减少过氧化氢相关的氧化损伤[66]抗乳腺癌[68]抗菌[70]镇痛、抗炎、抗病毒、抗真菌[72]延迟变色[75]保护心脏[79]抗癌[81]抗菌活性[84]N/A消炎[32]抗肝癌剂[32]10纳克/升空气[32]0.039 ppm溶于水[70]0.006 ppm溶于水[73]0.23 ppm溶于水[76]不符合21 ppm溶于水[82]12 ppm溶于水[84]18.8纳克/升空气[65]110.9纳克/升空气[62]665纳克/升空气[62]栀子花、番红花小苍兰、蔷薇、矮牵牛、大柱花三色堇菜、玫瑰番红花、小苍兰、藿香茴香、薰衣草蔷薇、鸡蛋花、金盏花和忍冬马鞭草、枳壳、花椒和天竺葵N/A
1.2.1 醇类
对于醇类挥发物,由于其低气味阈值,芳樟醇、香叶醇、2-苯乙醇、香茅醇和丁子香酚应在可食用花卉的加工中重点关注。橙花醇作为香叶醇的同分异构体也存在于许多具有抗菌活性的可食用花卉中[65]。然而,橙花醇的气味阈值高于香叶醇,因此主要含有橙花醇的可食用花卉不会像主要含有香叶醇的花卉那样具有浓烈的气味。尽管一些醇类挥发物在可食用花卉中的气味阈值低且浓度高,但它们的镇痛和麻醉特性限制了其日常摄入量[86]。因此,应考虑联合国粮食农业组织/世界卫生组织食品添加剂联合专家委员会(JECFA)的建议,可食用花卉产品中的挥发性化合物应被规范管理。相反,一些醇类挥发物作为功能性化合物应在可食用花卉的加工中予以保留。例如,含有较高香叶醇含量的玫瑰由于其较低的过氧化氢氧化损伤特性而具有较长的花卉储藏寿命[66]。
1.2.2 醛类
与醇类相比,醛类在可食用花卉中的存在较少,可能是因为它们不是可食用花卉挥发性途径的最终产物[24]。对于新鲜的可食用花卉,辛醛和己醛的气味活性高于其他醛类。在可食用花卉的加工过程中应重点留意己醛[87-88],因为它不仅出现在植物中,还作为关键香气出现在奶酪和煮熟的肉类中[89]。己醛通常由植物分泌,并被用于添加剂赋予各种食品青草味。此外,己醛的保留可能对延长可食用花卉的货架期有积极作用。Ashitha 等[75]指出,己醛可以抑制细胞壁降解酶,从而延缓花卉的变色和腐烂。因此,己醛的生成可能对可保存食用花卉的质量产生积极影响。在加工后,一些醛类会形成主要的风味物质,如番红花中的番红花醛。番红花醛从胡萝卜素氧化中产生但产量较少,因此新鲜花朵只具有轻微的番红花醛气味[77-78]。在加工后,番红花醛的含量增加,有助于产生清甜和绿植的香气。因此,番红花醛是一些可食用花卉的重要质量指标,例如小苍兰和藏红花。
1.2.3 酮类
由于其相对较高的挥发性,酮类挥发物在可食用花卉中只少量存在。Clarke[90]指出,酮类比具有相似分子量的其他化合物更具挥发性。然而,由于酮类是不断持续生成的产物,因此在可食用花卉中仍然存在,例如香叶酮和β-离子酮[82]。Fan等[83]报道了不同开花阶段的香叶酮含量,表明香叶酮可能在开花结束后仍然持续释放。β-离子酮由可食用花卉中的类胡萝卜素裂解双氧酶生成[80]。β-离子酮和香叶酮是可食用花卉浓烈果香的主要来源,并且已经在食品工业中应用了数个世纪[82,84]。
1.2.4 酯类
酯类的存在主要取决于可食用花卉中的萜类醇,因为它们是由乙酰转移酶(Acetyltransferase,AAT)酶运输的醇类合成的。多项研究表明,AAT酶可以激活醇类,用以合成可食用花卉中的乙酸酯[85]。因此,醇的含量和AAT 的活性影响酯的含量[64]。常见的酯有香叶醇、芳樟醇和香茅醇的乙酸酯,其香气强度较醇类低。香叶醇乙酸酯存在于玫瑰和薰衣草中,其气味被描述为花香、甜香和玫瑰香[65]。与香叶醇相比,香叶醇乙酸酯的气味阈值较高,但相关的香气强度较低。类似地,乙酸芳樟酯和乙酸香茅酯的气味阈值也较高[62]。尽管酯类不是醇类那样强烈的气味物质,但它们以一定数量存在,被认为是食品加工中安全的调味剂[91]。
1.2.5 可食用花卉挥发性物质的安全问题
植物衍生的挥发性化合物通常被普遍视为安全无害(Generally recognized as safe,GRAS)。然而,可食用花卉挥发性物质仍然存在一些潜在的安全风险。例如,丁香、茉莉和薰衣草的精油可能在长期接触下引发过敏反应。一些源自可食用花卉的挥发性物质被认为在低剂量下可供人类安全食用,但它们在食品产品中的实际含量可能要高得多[92]。目前,对于部分可食用花卉挥发性成分的日常摄入量只有一些初步的建议规定(表3)。因此,强化监管是解决可食用花卉挥发性物质安全问题的重要手段,特别需要在食品产品中维持有效剂量和潜在的风险之间找到平衡。
1.3 可食用花卉挥发性物质的合成和排放
在可食用花卉中,挥发性化合物在环境温度下具有低分子量和高蒸发气压的特点。这些挥发性物质由植物中光合固定的碳合成,并储存在植物的皮下细胞间隙,然后从花朵的表皮细胞中释放出来[57,93]。挥发性化合物的生物合成源自几个典型的生化途径,但在代谢途径中,挥发性物质的形成非常复杂。因此,挥发性物质的组成不完全相同,但在同一品种的花中却是相似的[57]。由于挥发性化合物在花朵中发挥生物学作用,挥发物的释放不仅由被动扩散决定,还受生物机制的控制[94]。合成的挥发性物质需要由一些载体蛋白和囊泡运输以跨越细胞障碍。这个过程维持了花朵开放阶段的挥发物的稳态排放,但在采摘后,可食用花卉中挥发性化合物的排放速率可能发生显著变化。
2 储存和加工对可食用花卉挥发性物质的影响
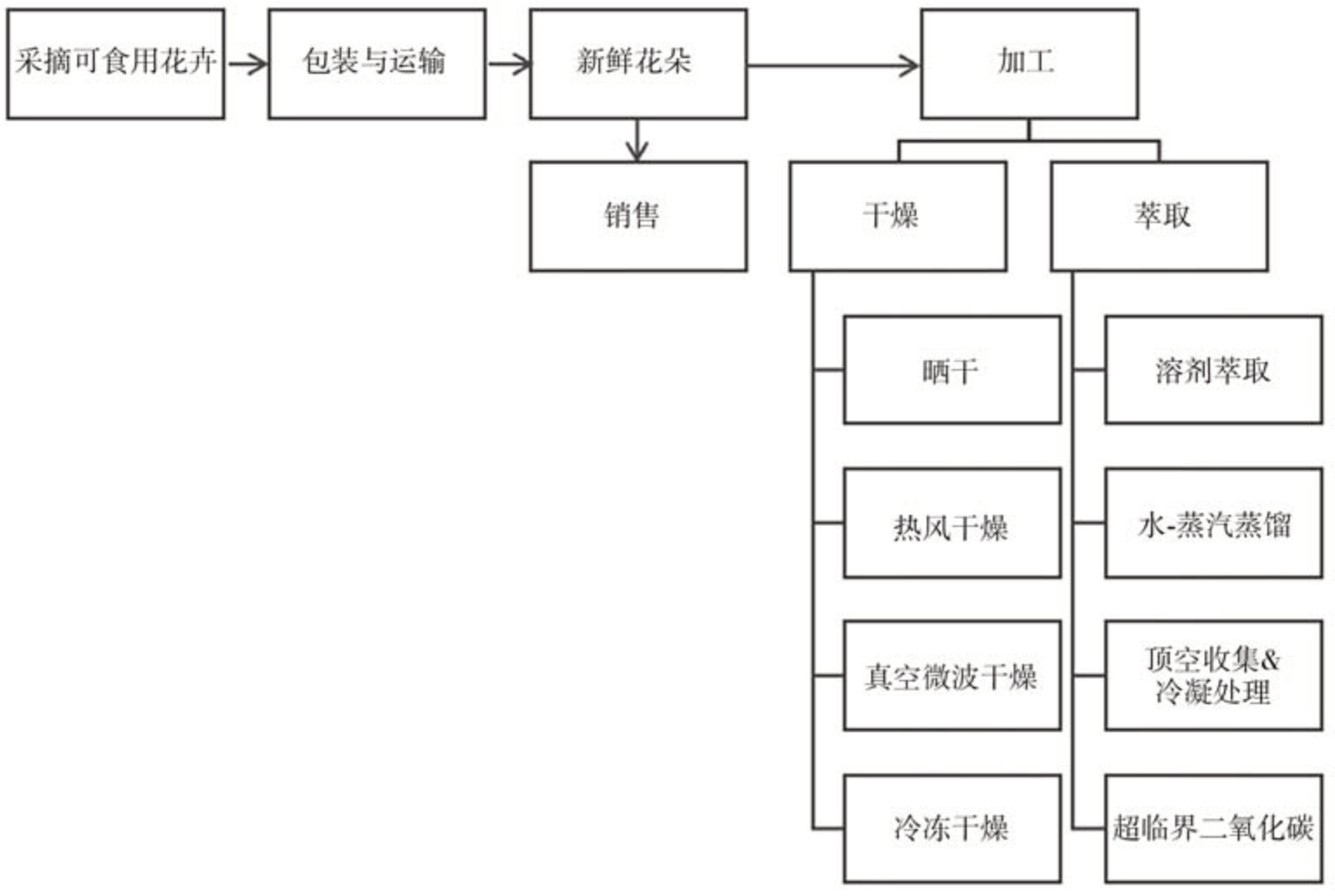
可食用花卉的常见加工流程,包括包装、储存和加工,如图1所示。由于可食用花卉的挥发物含量受地理位置、采摘时间等因素的影响,不同研究描述同一物种的结果不好定量比较。因此,挥发性化合物的反应及其在储存、干燥和萃取过程中的含量变化是关注的重点。部分具有代表性的可食用花卉中主要挥发性化合物的变化见表4。储存技术、干燥技术和萃取技术对可食用花卉香气的影响将在下面的小节中讨论。
表4 不同加工方法下可食用花卉主要挥发性成分的变化
Table 4 Change of main volatiles of edible flowers under different processing methods

品种参考文献木槿[30]挥发物新鲜晒干[29]挥发物晒干后98℃热水冲泡16分钟晒干,25 ℃冷泡24小时芳樟醇1-辛烯-3-醇L ND ND苯甲醇3-戊烯-2-醇壬醛2-甲基-3-丁烯-2-醇己醛2-糠醛(E,E)2,4-壬二烯醛2-壬醛癸醛2-辛烯醛辛醛香叶基丙酮L L ND[33]风干并98 ℃提取30分钟(Z)-3-己烯-1-醇1-辛烯-3-醇2-甲氧基-4-乙烯基苯酚己醛糠醛己醛糠醛L ND ND ND 5-甲基-2-糠醛壬醛α-紫罗兰酮β-紫罗兰酮5-甲基-2-呋喃酮香叶基丙酮1-辛烯-3-酮1-辛烯-3-酮3-辛酮L L L L D H H D H D D D H H H H 2-戊基呋喃苯乙醛丁子香酚5-甲基-2-糠醛壬醛3-甲基-3-丁烯-2-酮苯甲醛γ-丁内酯泛内酯3-羟基-3-甲基-2-丁酮D-柠檬烯2-糠酸甲酯醋酸丁子香酚2-甲基丁酸2-甲酰基吡咯H S L L L L L S L L S L L L L L H H L S H H H H H S H H S H H H H H L L品种参考文献狭叶薰衣草[19]挥发物芳樟醇龙脑橙花醇4-萜品醇1-辛烯-3-醇新鲜60 ℃对流干燥70 ℃对流干燥水蒸气蒸馏L VL VL VL L L L L V真空微波干燥(功率480 W)VH VH超临界二氧化碳L ND乙酸芳樟酯L L乙酸香叶酯桉树脑L H VL L H L L H H L L L H L L H H VH VL L[21]挥发物芳樟醇龙脑橙花醇萜品醇香叶醇1-辛烯-3-醇乙酸酯乙酸芳樟酯乙酸香叶酯β-石竹烯香豆素7-甲氧基香豆素α-松油醇H H H H H S L L H ND ND H L L L S H L L L L H
续表4
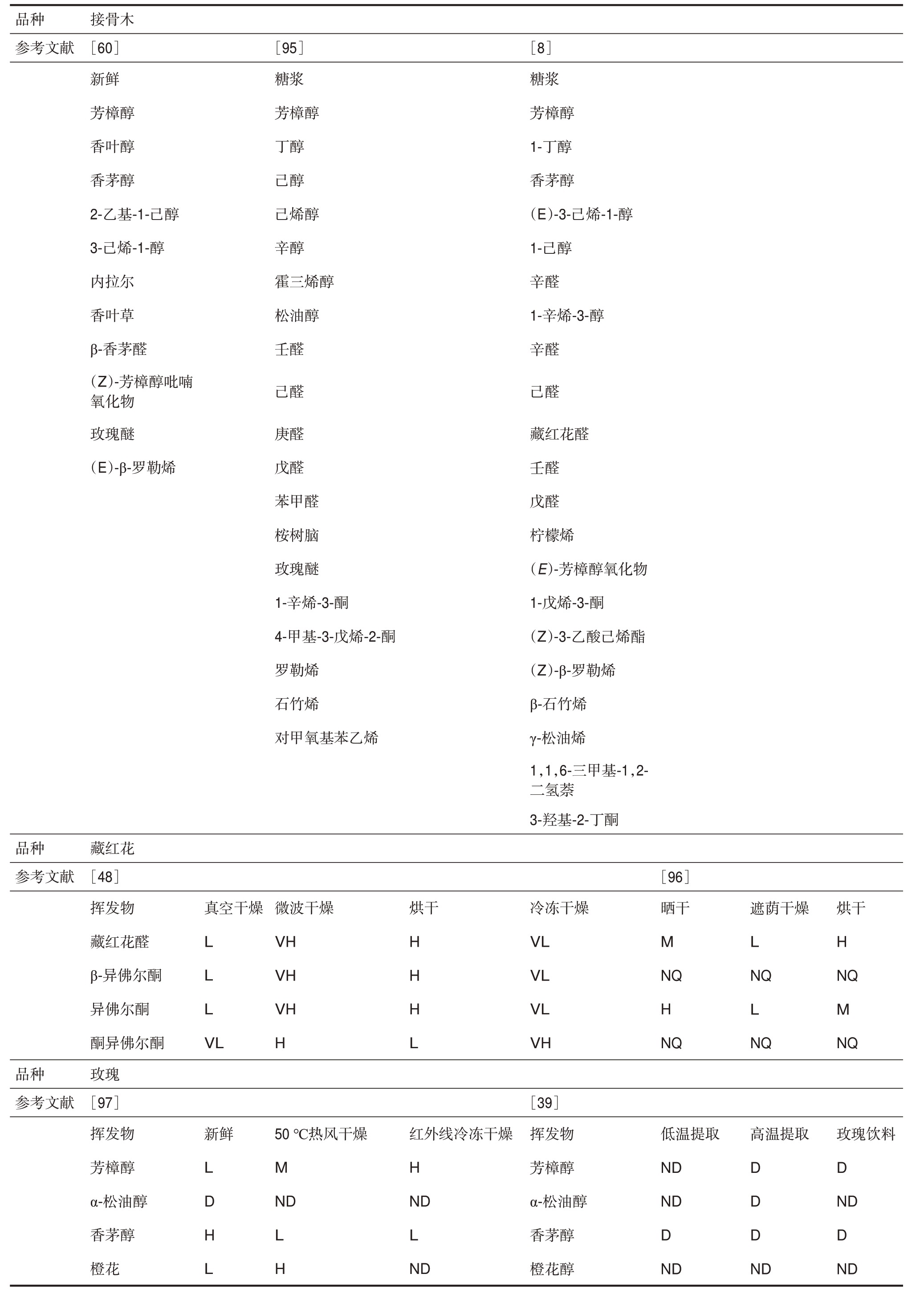
品种参考文献接骨木[60]新鲜芳樟醇香叶醇香茅醇2-乙基-1-己醇3-己烯-1-醇内拉尔香叶草β-香茅醛(Z)-芳樟醇吡喃氧化物玫瑰醚(E)-β-罗勒烯[95]糖浆芳樟醇丁醇己醇己烯醇辛醇霍三烯醇松油醇壬醛[8]糖浆芳樟醇1-丁醇香茅醇(E)-3-己烯-1-醇1-己醇辛醛1-辛烯-3-醇辛醛己醛己醛庚醛戊醛苯甲醛桉树脑玫瑰醚1-辛烯-3-酮4-甲基-3-戊烯-2-酮罗勒烯石竹烯对甲氧基苯乙烯藏红花醛壬醛戊醛柠檬烯(E)-芳樟醇氧化物1-戊烯-3-酮(Z)-3-乙酸己烯酯(Z)-β-罗勒烯β-石竹烯γ-松油烯1,1,6-三甲基-1,2-二氢萘3-羟基-2-丁酮品种参考文献藏红花[48]挥发物藏红花醛β-异佛尔酮异佛尔酮酮异佛尔酮玫瑰[97]挥发物芳樟醇α-松油醇香茅醇橙花真空干燥微波干燥VH VH VH H烘干L L L VL H H H L冷冻干燥VL VL VL VH[96]晒干M NQ H NQ遮荫干燥L NQ L NQ烘干H NQ M NQ品种参考文献新鲜高温提取L D H L 50 ℃热风干燥M ND L H红外线冷冻干燥H ND L ND[39]挥发物芳樟醇α-松油醇香茅醇橙花醇低温提取ND ND D ND D D D ND玫瑰饮料D ND D ND
续表4

注: D=检测到; H=相对较高的气味强度; L=相对较低的气味强度; M=相对中等的气味强度; ND=未检测到; NQ=未量化; S=相同的气味强度水平; VH=相对最高的气味强度水平; VL=相对最低的气味强度水平。
Note: D=detected; H=relatively high level of odour intensity; L=relatively low level of odour intensity; M=relatively medium level of odour intensity; ND=not detected; NQ=not quantified; S=same level of odour intensity; VH=relatively highest level of odour intensity; VL=relatively lowest level of odour intensity.
品种参考文献玫瑰[97]橙花香叶醇苯乙醇丁子香酚己醛壬醛L L D D ND H H ND ND ND ND ND D D ND D D D ND D D N ND D D ND L ND ND L ND[39]橙花醇香叶醇乙醇橙花叔醇甲基丁香酚丁子香酚香叶酸甲酯乙酸香茅酯十七烷正二十烷十九烷β-月桂烯D-柠檬烯玫瑰醚ND D ND D ND ND ND D ND D D ND D ND ND ND ND D D D ND D ND ND ND ND ND D D D
2.1 储存技术对可食用花卉挥发性物质的影响
在可食用花卉中应用适宜的储存技术可以通过延长货架寿命和降低蒸发速率来保留更多原始的挥发性化合物。将可食用花卉储存于0~4 ℃被认为是降低呼吸速率和蒸发速率的主要控制方法[16,98]。低呼吸速率延长了可食用花卉的货架寿命,允许挥发性化合物持续合成和积累。呼吸速率越高,货架寿命越短,因此可食用花卉中的挥发物含量越低,原因可能是花朵中的生物合成反应停止了[99]。由于可食用花卉是在开花阶段采摘,挥发性化合物可以通过上述的生物合成途径合成和积累。当可食用花卉寿命结束时,生物调节停止,因此挥发物含量将迅速减少[100]。此外,高蒸发率也会导致可食用花卉的挥发物流失。在升高的温度条件下,挥发性化合物的释放速率会加速[1]。高温也是促进呼吸速率升高的主要因素之一。大多数酶在冷藏条件下活性较低,这可以延缓可食用花卉的衰老并保持挥发性化合物[101]。值得注意的是,在这种储存条件下,萜类挥发物的保存要好得多[102]。
此外,采用适当的包装材料进行真空保存是延长已采摘可食用花卉货架寿命的另一种方法。在储存过程中,切花暴露在空气中,并受到采摘后压力的影响,如氧化应激物质(ROS)的产生增加。ROS 的生成会导致可食用花卉中的蛋白质和脂质受损[101]。这可能会加速可食用花卉的腐烂并引起香气特性的变化。根据Kaack 等[11]的研究,当包装在纸袋而不是塑料袋中时,接骨木花会呈现更具特色的青草香味。当暴露于空气中时,接骨木花会生成包括戊醛、己醛、庚醛、戊基呋喃、辛醛、辛二烯醛在内的醛类化合物。为避免风味变化,可食用花卉可以储存在真空铝箔袋中。Salvador等[102]和Kaack等[11]的研究都报道了类似的结果,即接骨木花在真空包装下的原始香气比其他包装方法更多。事实上,储存时蒸发速率低的可食用花卉可以保持更多挥发性化合物。低密度聚乙烯作为包装材料也可以用于可食用花卉的储存。有研究表明,由于其对醇类和醛类的屏障特性,可食用花卉在低密度聚乙烯包装下呈现出较低的蒸发速率而具有较长的货架期[103]。
气调包装(Modified atmosphere packing,MAP)也是延长可食用花卉货架寿命和保持挥发物含量的有效方法。Wang等[104]的研究表明,在氢气下储存可食用花卉可以延长其货架寿命。这是因为氢气可以降低特定酶(1-氨基环丙烯-1-羧酸合成酶)的活性,以防止乙烯的生物合成[104]。没有乙烯,可食用花卉的衰老速度会降低,因此香气化合物可以被保留。然而,使用乙醇而不是氢气的气调包装方法对可食用花卉的储存不起正面作用[105]。因此,将氢气包装与冷藏相结合可能是可食用花卉的实际储存方法。
2.2 干燥技术对可食用花卉挥发性物质的影响
干燥是保存可食用花卉常见采摘后的操作方法。对于可食用花卉的挥发性化合物来说,温度在各种干燥技术中起着重要作用。在高温下,各种干燥过程会导致可食用花卉中新的挥发性化合物生成。晾晒和热风干燥是传统方法,但微波干燥可以防止花朵收缩。就冷冻干燥而言,干燥后的花朵与新鲜花朵具有相似的风味,表明其是一种良好的干燥方法[106]。然而,与新鲜采摘的花朵相比,所有的干燥过程都会增加可食用花卉的总挥发物含量。这可能是因为干燥过程破坏了可食用花卉的细胞屏障,使更多的挥发性化合物易于通过表皮细胞传递到大气中[57]。
2.2.1 对醇类挥发物的影响
在干燥处理后,可食用花卉中来自醇类挥发物的大多数脂肪酸的含量会增加,而少数醇类会氧化。在较高温度下干燥可能会导致可食用花卉中醇类含量的增加,这可能是由于高温下生物合成过程被加速。在高温下,各种酶的活性增加,以分解其底物和脂质产生更多的醇类挥发物[57,94]。例如,亚油酸是可食用花卉中常见的酸类,它在热处理下可以被脂氧合酶和羟基过氧化物酶分解,从而生成1-辛烯-3-醇[6,107]。Juhari 等[24]和Ramirez 等[31]都证明,在各种干燥处理后,芙蓉花中的1-辛烯-3-醇含量显著增加。Lyczko 等[19]显示,薰衣草花在70 ℃的对流干燥下含有更多的醇类,包括莰醇、蓬勃醇和1-辛烯-3-醇,而在高温下,带有更高干燥动力学的真空-微波(480 V)干燥可以在薰衣草中生成更多的醇类。此外,Fancello 等[96]报告说,烤炉干燥的藏红花花朵比晒干和遮荫干燥的花朵含有更多的醇类。相反,在冷冻干燥条件下,可食用花卉中醇类挥发物的增加微不足道。Qiu等[97]比较了冷冻干燥和热风干燥对玫瑰花挥发性化合物的影响,结果发现冷冻干燥的玫瑰花中脂肪醇的含量较低。然而,一些特定的醇类在高温下会减少,例如香茅醇和苯乙醇,这可能是由于在干燥过程中这些醇类挥发物发生氧化反应[31]。此外,在干燥的可食用花卉中,莰醇的含量也不相同。在经过干燥处理后,薰衣草和玫瑰花的莰醇含量增加,而芙蓉花的莰醇含量下降。这可能是因为莰醇在可食用花卉中经历了生成和热降解两种情况,具体变化取决于花朵的种类[19,24,97]。
2.2.2 对醛类和酮类挥发物的影响
热风干燥过程会导致各种酮类和醛类的生成。在热处理下,美拉德反应、碳水化合物脱水和类胡萝卜素裂解可能会产生新的醛类和酮类,为可食用花卉带来甜美和烘焙香气[108]。与晾晒相比,热风干燥和微波干燥由于使用较高的温度,表现出更好的干燥性能。随着湿度降低,这些反应在干燥过程中需要更多的活化能[109]。例如,烤箱干燥的洛神花含有比晾晒洛神花更高水平的Strecker 醛(2-甲基丁醛和3-甲基丁醛)和糠醛[24]。Strecker 醛是由美拉德反应中氨基酸的脱羧反应形成的,并由于氨基酸侧链的功能基团而显得复杂[89]。分支链醛在干燥的芙蓉花中也会形成[24,110]。此外,线性醛来自可食用花卉中脂肪酸的氧化。在玫瑰花烘干后,脂肪醛如癸醛、辛醛和己醛会作为新的香气活性化合物生成[97]。这些挥发性化合物也被发现在干燥的接骨木花中[60]。
此外,干燥过程会导致在烯丙醇化和脱水中形成单糖,从而增加了醛类挥发物的含量[31,89]。在洛神花中,生成最多的醛类是糠醛,它是由脱水五碳糖如木糖和阿拉伯糖产生的。糠醛常常被观察到与丙酮伴随出现[31]。因此,也有人提出抗坏血酸可能被降解为酮和它的还原产物,包括糠醛[111]。在热处理过程中,藏红花中的番红花素是通过类胡萝卜素裂解双氧酶(Carotenoid cleavage dioxygenases,CCD)形成的,它们裂解类胡萝卜素的多重双键[112]。随着温度的升高,2,6,6-三甲基-4-羟基-1-羧醛-1-环己烯(HTCC)和菠菜素分别作为前体逐渐转化为番红花素。对于番红花来说,番红花素是衡量其品质的主要指标,因此干燥是提高其番红花素含量的主要方法。此外,类胡萝卜素裂解还导致了许多可食用花卉中各种酮类的生成,特别是β-离子。β-胡萝卜素被CCD家族成员CCD1催化氧化裂解以形成β-紫罗兰酮[113]。在干燥过程中,氧气含量可能也会影响酮类的生成,而低氧气的干燥过程可能对酮类的气味活性值产生积极影响,如真空干燥或微波干燥[19,113-114]。
2.2.3 对酯类挥发物的影响
可食用花卉中酯类挥发物的含量会受到干燥加工中各种因素的影响。一些酯类,如乙酸芳樟醇酯和乙酸尼罗利酮酯,在热风干燥期间会减少,但在晾晒期间会增加。这些酯类在热风干燥过程中可能容易受热降解生成醇和酸[19,31]。相反,这些酯类也可能通过酶催化的酯交换反应而合成。Shaimaa 等[115]和Aprotosoaie 等[86]描绘了芳樟醇乙酸酯的代谢途径,可以通过相关酶将葡萄糖醛酸与芳樟醇结合而形成。在接骨木花中,总的芳樟醇乙酸酯含量在冷冻干燥下明显减少,而在冷冻时几种酶的活性可能是影响酯类含量的主要因素,而这又会进一步受到可见光、氧气和水分活性的影响[102]。
2.3 萃取技术对可食用花卉挥发性物质的影响
萃取是一种将挥发性化合物从可食用花卉中分离出来的过程,从而制备挥发性化合物含量高的提取物。由于可食用花卉提取物通常应用于食品工业中的饮料制备,因此水和二氧化碳被视为常见的提取溶剂,这样可以避免杂质和不良气味的出现[16]。大多数挥发性化合物是亲水性的,因此干燥技术可以用作萃取过程的预处理。在干燥的可食用花卉中,细胞组织的破坏使得挥发性化合物可以轻松地穿越细胞屏障进入溶剂中。因此,在干燥的花卉提取物中可以检测到比新鲜花卉提取物中更多的挥发性化合物。Lyczko 等[19]指出,使用相同的萃取技术,干燥的薰衣草的提取产率(9.8%)高于新鲜薰衣草(0.51%)。由于萃取的不同预处理可能会影响挥发性化合物的组成,因此对同一种类别的食用花卉进行不同研究可能存在显著性差异。例如,与阳光干燥的洛神花提取物相比,热风干燥的洛神花提取物包含不同的醇类挥发物,但也包括呋喃甲醛、壬醛和己醛等相似的醛类挥发物[29,32]。此外,其他因素也可能影响挥发性化合物,包括新鲜或干燥花卉、溶剂、提取时间和提取温度[27]。
与冷浸泡相比,热浸泡会在花卉中产生更多的醇类化合物。在玫瑰花提取时,高温提取物中通常检测到有香茅醇、α-松油醇和奈洛利醇,而在冷温提取物中通常不会检测到。Kiran 等[21]也得出了相同的结论,使用水蒸馏和水蒸汽蒸馏的薰衣草提取物中的醇类含量高于相对较低温度的超临界二氧化碳提取。这可能是因为在高温下,可食用花卉中的酯类化合物容易水解为相应的酸和醇[21]。然而,Zannou等[29]在98 ℃处理16 min的洛神花挥发物提取物中显示,尤甘醚和2-甲基-3-丁烯-2-醇的含量较低,与在25 ℃处理24 h的提取物相比,3-戊烯-2-醇的含量相似。因此,提取时间也是一个需要考虑的关键因素。对于醛类化合物,其变化趋势与醇类类似。在冷浸泡24 h 的洛神花挥发物提取物中,呋喃甲醛、壬醛和己醛的含量较低,而在热浸泡16 min 的提取物中较高,但2-甲基吡咯酮的含量在较高温度下更高。Shi等[116]指出,当热和质量传递同时发生时,各种醛类化合物可能会通过生物活性化合物的分解而生成。这种化学反应类似于干燥过程。壬醛和己醛是洛神花和接骨木花干燥提取物中检测到的主要醛类化合物[29,95]。在萃取过程中,有许多因素会影响可食用花卉的挥发性化合物。因此,一些新的提取技术被开发出来以提高提取效率。例如,Zhou 等[16]扩大了挥发物的收集和冷凝处理规模来收集和分离茉莉花中的挥发性化合物,从而使挥发性提取物的香气特性与新鲜花卉的相似。
3 当前研究的局限性
可食用花卉中的挥发性化合物种类繁多且容易与其他物质反应。适当的加工技术应用对于保留可食用花卉中的香气化合物至关重要。尽管在可食用花卉的加工过程中已应用了一些新的技术,但对其挥发性化合物的影响研究仍然有限。许多研究仅展示了可食用花卉中总挥发物的含量,而没有通过嗅觉分析来确定香气活性挥发物。这可能会阻碍随后的研究进展,因为只有香气活性挥发物才能显著影响可食用花卉的香气特性。此外,气相色谱-嗅觉法仍存在一些局限性,可能无法鉴定可食用花卉中的某些挥发物。关于主要挥发性化合物萜烯,大多数研究并未讨论可食用花卉加工对它们以及相关香气的影响。关于一些具有浓郁香气的商业花卉,如桂花和茉莉花,不同加工条件对其的影响仍然没有相关的报告。
4 结论
本文讨论了一些具有浓郁香气的代表性可食用花卉,包括玫瑰属、薰衣草、黑接骨木、洛神花和藏红花。由于它们的香气特性,这些新鲜或加工后的玫瑰花瓣和洛神花萼、藏红花干雄蕊、薰衣草油和接骨木花提取物被广泛应用于食品制品中。尽管可食用花卉中能释放出数千种挥发性化合物,但只有香气活性化合物主要影响香气特性。这些化合物主要分为醇、醛、烯烃和酯类。在可食用花卉加工过程中,应考虑挥发性化合物的安全问题,这对于可食用花卉中挥发物的释放,被动扩散和生物调控起着重要作用。在可食用花卉的储存和加工过程中,挥发性化合物都会有显著变化。为了保持原有挥发物含量,可以使用气调包装在0~4 ℃下储存新鲜可食用花卉,从而降低可食用花卉的呼吸速率和挥发物排放速率。为增加挥发性化合物的含量,干燥技术可以破坏细胞壁,释放更多挥发物到大气中,从而丰富可食用花卉的香气特性。高温下的晒干、热风干燥和微波干燥可能会导致脂肪酸降解、美拉德反应、类胡萝卜素裂解,以增加醇含量并生成具有烘焙香气的各种醛。萃取是获得可食用花卉挥发性化合物的技术,但会破坏花朵的外观。在萃取过程中,许多因素可能会影响挥发性化合物的含量,包括新鲜或干燥的花朵、溶剂、添加剂、提取时间和提取温度。萃取后,由于酯的水解、生物活性化合物的分解以及溶剂中的其他添加剂,醇、醛和酮的含量会增加。一些新开发的技术,如顶空收集和冷凝处理,会保留更多的原始挥发物并生产出与新鲜可食用花卉类似的高质量提取物。根据储存和加工技术对挥发性化合物的影响,可食用花卉的香气特性可以得到增强或保持。
可食用花卉在食品工业中仍然是一个新兴领域,未来需要进一步研究探索不同加工技术对具有明显商业前景的可食用花卉的影响,为丰富花卉食品开发提供技术参考。
参考文献:
[1] TAKAHASHI J A, REZENDE F A G G, MOURA M A F, et al.Edible flowers: Bioactive profile and its potential to be used in food development[J]. Food Research International, 2020, 129,108868.
[2] FERNANDES L, CASAL S, PEREIRA J, et al. An overview on the market of edible flowers[J]. Food Reviews International,2019, 36(3): 258-275.
[3] ZHAO G, KUANG G, LI J, et al. Characterization of aldehydes and hydroxy acids as the main contribution to the traditional Chinese rose vinegar by flavor and taste analyses[J]. Food Research International, 2020, 129, 108879.
[4] FERNANDES L, CASAL S, PEREIRA J A, et al. Borage,calendula, cosmos, Johnny Jump up, and pansy flowers:volatiles, bioactive compounds, and sensory perception[J]. European Food Research and Technology, 2019: 593-606.
[5] FERNANDES L, CASAL S, PEREIRA J, et al. Edible flowers:A review of the nutritional, antioxidant, antimicrobial properties and effects on human health[J]. Journal of Food Composition and Analysis, 2017, 60: 38-50.
[6] FERNANDES L, RAMALHOSA E, PEREIRA J, et al. The unexplored potential of edible flowers lipids[J]. Agriculture,2018, 8(10).
[7] PIRES T C S P, DIAS M I, BARROS L, et al. Edible flowers as sources of phenolic compounds with bioactive potential[J]. Food Research International, 2018: 580-588.
[8] KAACK K. Processing of aroma extracts from elder flower(Sambucus nigra L.) [J]. European Food Research and Technology, 2008, 227(2): 375-390.
[9] KAMENJAKOVIC M, HADZIC V, KULIC S, et al. Influence of preservatives on quality elderflower syrup (Sambucus nigra L.)[J]. Food in Health and Disease, 2017, 1-6.
[10] SEDLACKOVA V H, GRYGORIEVA O, FATRCOVASRAMKOVA K, et al. The morphological and antioxidant characteristics of inflorescences within wild-growing genotypes of elderberry (Sambucus nigra L.) [J]. Potravinarstvo Slovak Journal of Food Sciences, 2018, 444-453.
[11] KAACK K, CHRISTENSEN L P. Effect of packing materials and storage time on volatile compounds in tea processed from flowers of black elder (Sambucus nigra L.) [J]. European Food Research and Technology, 2008, 227(4): 1259-1273.
[12] COLDEA T, MUDURA E, POP C, et al. Fermentation of an aromatized wine-based beverage with sambucus nigra l. syrup(after champenoise method) [J]. Food Science and Technology,2015, 72(2).
[13] ŚLⅠWⅠŃSKA M, WⅠŚNⅠEWSKA P, DYMERSKI T, et al.Authenticity assessment of the “onisiówka” nalewka liqueurs using two-dimensional gas chromatography and sensory evaluation [J]. Food Analytical Methods, 2016, 10(6): 1709-1720.
[14] ISAC X A, RAJADURAI K R, JAWAHARLAL M, et al. Aroma profiling of jasmine (Jasminum sambac Ait.) flowers using electronic nose technology[J]. Journal of Applied Horticulture,2016: 19-24.
[15] ZHANG J, LI J, WANG J, et al. Characterization of aromaactive compounds in Jasminum sambac concrete by aroma extract dilution analysis and odour activity value[J]. Flavour and Fragrance, 2020.
[16] ZHOU H C, HOU Z W, WANG D X, et al. Large scale preparation, stress analysis, and storage of headspace volatile condensates from Jasminum sambac flowers[J]. Food Chemistry, 2019, 286: 170-178.
[17] ITO Y, SUGIMOTO A, KAKUDA T, et al. Identification of potent odorants in Chinese jasmine green tea scented with flowers of Jasminum sambac[J]. Journal of Agricultural and Food Chemistry, 2002: 4878-4884.
[18] SONMEZDAG A S, KELEBEK H, SELLI S. Identification of aroma compounds of Lamiaceae species in Turkey using the purge and trap technique[J]. Foods, 2017, 9.
[19] LYCZKO J, JALOSZYNSKI K, SURMA M, et al.Determination of various drying methods' impact on odour quality of true lavender (Lavandula angustifolia Mill.) Flowers[J]. Molecules, 2019, 24(16).
[20] ÖZOGUL F, TUGCE AKSUN E, ÖZTEKIN R, et al. Effect of lavender and lemon balm extracts on fatty acid profile, chemical quality parameters and sensory quality of vacuum packaged anchovy (Engraulis encrasicolus) fillets under refrigerated condition[J]. Lwt, 2017, 84: 529-535.
[21] KIRAN D, SHARMA A, SINGH B. Volatile composition of Lavandula angustifoliaproduced by different extraction techniques[J]. Journal of Essential Oil Research, 2016, 28(6):489-500.
[22] FUKUSHIMA S, COHEN S M, EISENBRAND G, et al. FEMA GRAS assessment of natural flavor complexes: Lavender,Guaiac Coriander-derived and related flavoring ingredients[J].Food Chemical Toxicology, 2020, 145, 111584.
[23] MILEA S A, DIMA C V, ENACHI E, et al. Combination of freeze drying and molecular inclusion techniques improves the bioaccessibility of microencapsulated anthocyanins from black rice (Oryza sativa L.) and lavender (Lavandula angustifolia L.)essential oils in a model food system[J]. International Journal of Food Science & Technology, 2020: 3585-3594.
[24] JUHARI N H, BREDIE W L P, TOLDAM-ANDERSEN T B,et al. Characterization of Roselle calyx from different geographical origins[J]. Food Research International, 2018, 112: 378-389.
[25] HONG X, WANG L X, CHEN Z W, et al. Study on the processing technology of roselle angel cake[J]. Food Research and Development, 2017, 38(18), 82-86.
[26] IDOLO I, MARSHALL L J. The effect of ageing temperature on the sensory qualities of Hibiscus sabdariffa (Roselle) wine[J].African Journal of Food, Agriculture, Nutrition and Development, 2019: 14726-14738.
[27] IFIE I, LISA J M, PETER H, et al. Hibiscus sabdariffa (Roselle)extracts and wine: Phytochemical profile, physicochemical properties, and carbohydrase inhibition[J]. Journal of Agricultural and Food Chemistry, 2016, 64(24): 4921-4931.
[28] ALMANA H A. Karkade (Hibiscus sabdariffa) as a mineral and fiber supplement in chocolate cakes[J]. Arab Universities Journal of Agricultural Sciences (Egypt), 2001, 9(1), 283-295.
[29] ZANNOU O, KELEBEK H, SELLI S. Elucidation of key odorants in Beninese Roselle (Hibiscus sabdariffa L.) infusions prepared by hot and cold brewing[J]. Food Research International, 2020.
[30] RAMIREZ M M, PLAZA M L, AZEREDO A, et al.Phytochemical, sensory attributes and aroma stability of dense phase carbon dioxide processed Hibiscus sabdariffa beverage during storage[J]. Food Chem, 2012, 134(3): 1425-1431.
[31] RAMIREZ M M, BALABAN M O, MARSHALL M R, et al.Hot and cold water infusion aroma profiles of Hibiscus sabdariffa: fresh compared with dried[J]. Journal of Food Science,2011: 212-217.
[32] ELSHARIF S A, BUETTNER A. Influence of the chemical structure on the odor characters of beta-citronellol and its oxygenated derivatives[J]. Food Chemstry, 2017, 232: 704-711.
[33] AVALOS-MARTINEZ E, PINO J A, SAYAGO-AYERDI S, et al.Assessment of volatile compounds and sensory characteristics of Mexican hibiscus (Hibiscus sabdariffa L.) calyces hot beverages[J]. Journal of Food Science and Technology, 2019: 360-366.
[34] WANG J H, ZHANG L L, WANG C H, et al. The development of compound jam-liand chrysanthemum[J]. China Condiment,2019, 44(4), 113-115.
[35] ARSLANER A, SALIK M A, BAKⅠRCⅠ İ. The effects of adding Hibiscus sabdariffa L. flowers marmalade on some quality properties, mineral content and antioxidant activities of yogurt[J]. Journal of Food Science and Technology, 2020, 1.
[36] SUN, YE Z, LI J, et al. Effect of the addition of roselle (Hibiscus sabdariffa L.) extracts on the rheological, textural, and antioxidant activity of fermented milks[J]. Flavour and Fragrance Journal, 2020: 42-50.
[37] KIM J H, CHA J Y, SHIN T S, et al. Volatile flavor components of blended tea with fermented tea and herbs[J]. Preventive Nutrition and Food Science, 2018, 23(3): 245-253.
[38] FARAG M A, RASHEED D M, KAMAL I M. Volatiles and primary metabolites profiling in two Hibiscus sabdariffa (roselle)cultivars via headspace SPME-GC-MS and chemometrics[J]. Food Research International, 2015, 78: 327-335.
[39] ZHAO C Y, XUE J, CAI X D, et al. Assessment of the key aroma compounds in rose-based products[J]. Journal of Food and Drug Analysis, 2016, 24(3): 471-476.
[40] QIU L, ZHANG M, JU R, et al. Effect of different drying methods on the quality of restructured rose flower (Rosa rugosa)chips[J]. Drying Technology, 2019, 38(12): 1632-1643.
[41] ZHANG C, ZHAO F, LI R, et al. Purification, characterization,antioxidant and moisture-preserving activities of polysaccharides from Rosa rugosa petals[J]. International Journal of Biological Macromolecules, 2019, 124, 938-945.
[42] HNIN K K, ZHANG M, DEVAHASTIN S, et al. Influence of novel infrared freeze drying of rose flavored yogurt melts on their physicochemical properties, bioactive compounds and energy consumption[J]. Food and Bioprocess Technology, 2019,12(12): 2062-2073.
[43] LIU T, WANG Q, FENG Z, et al. The production of rose flower cake and its process optimization[J]. Food Industry, 2020:110-114.
[44] LI M, QIN C, HANG Y, et al. Development of rose jelly[J]. Storage and Process, 2018: 120-124.
[45] ZHAO J, XU F, HUANG H, et al. Evaluation on bioactivities of total flavonoids from Lavandula angustifolia[J]. Pakistan Journal of Pharmaceutical Sciences, 2015, 28(4): 1245-1251.
[46] JOICHI A, NAKAMURA Y, HAZE S, et al. Volatile constituents of blue-coloured hybrid tea rose flowers[J]. Flavour and Fragrance Journal, 2013, 28(3): 180-187.
[47] ZHOU L, YU C, CHENG B, et al. Volatile compound analysis and aroma evaluation of tea-scented roses in China[J]. Industrial Crops & Products, 2020, 155.
[48] CHEN D, XING B, YI H, et al. Effects of different drying methods on appearance, microstructure, bioactive compounds and aroma compounds of saffron (Crocus sativus L.)[J]. LWTFood Science and Technology, 2020, 120.
[49] FEYZI S, VARIDI M, HOUSAINDOKHT M R, et al. A study on aroma release and perception of saffron ice cream using invitro and in-vivo approaches[J]. Innovative Food Science and Emerging Technologies, 2020, 65.
[50] ALMODOVAR P, PRODANOV M, ARRUÑADA O, et al.Affron eye, a natural extract of saffron (Crocus sativus L.) with colorant properties as novel replacer of saffron stigmas in culinary and food applications[J]. International Journal of Gastronomy and Food Science, 2018, 12: 1-5.
[51] AMANPOUR A, SONMEZDAG A S, KELEBEK H, et al. GCMS-olfactometric characterization of the most aroma-active components in a representative aromatic extract from Iranian saffron (Crocus sativus L.)[J]. Food Chemistry, 2015: 251-256.
[52] DONG C, WANG X, LI Y, et al. Response surface methodology for optimizing fermentation technology of Osmanthus fragrans honey wine[J]. Food Industry, 2020: 81-84.
[53] SHENG X, LIN Y, CAO J, et al. Comparative evaluation of key aroma-active compounds in sweet osmanthus (Osmanthus fragrans Lour.) with different enzymatic treatments[J]. Journal of Agricultural and Food Chemistry, 2020, 69(1), 332-344.
[54] LUO D, CHEN J, GAO L, et al. Geographical origin identification and quality control of Chinese chrysanthemum flower teas using gas chromatography-mass spectrometry and olfactometry and electronic nose combined with principal component analysis[J]. International Journal of Food Science &Technology, 2017: 714-723.
[55] LU Q, XUE S, YANG D, et al. Comparative analysis of flavor quality of chrysanthemum tea (Chrysanthemum morifolium cv.“Fubaiju”) processed by different drying methods[J]. Food Science,China, 2020: 249-255.
[56] LIM T K. Edible medicinal and non-medicinal plants[M]. Springer,2014.
[57] PICHERSKY E, DUDAREVA N A. Biology of plant volatiles (Second edition.) [J]. CRC Press, 2020.
[58] OHASHI T, ISHIZAKI S, KUROBAYASHI Y, et al. Identification of odor-active trace compounds in blooming flower of damask rose (Rosa damascena)[J]. Journal of Agricultural and Food Chemistry, 2019, 67(26): 7410-7415.
[59] ZOU J, CAI X, XIANG Z, et al. Characterization of aromaactive compounds from sweet osmanthus (Osmanthus fragrans)by SDE and SPME coupled with GC-MS and GC-olfactometry[J]. International Journal of Agriculture and Biology, 2019:277-282.
[60] BAJER T, BAJEROVA P, VENTURA K. Effect of harvest and drying on composition of volatile profile of elderflowers(Sambucus nigra) from Wild[J]. Natural Product Communications,2017, 12(12): 1937-1942.
[61] CULLERE L, SAN-JUAN F, CACHO J. Characterisation of aroma active compounds of Spanish saffron by gas chromatography-olfactometry: quantitative evaluation of the most relevant aromatic compounds[J]. Food Chemistry, 2011:1866-1871.
[62] ELSHARIF S A, BANERJEE A, BUETTNER A. Structure-odor relationships of linalool, linalyl acetate and their corresponding oxygenated derivatives[J]. Frontiers Chemistry, 2015, 3, 57.
[63] XU Y, TONG Z, ZHANG X, et al. Unveiling the mechanisms for the plant volatile organic compound linalool to control gray mold on strawberry fruits[J]. Journal Agricutural Food Chemistry, 2019, 67(33): 9265-9276.
[64] SHALIT M, GUTERMAN I, VOLPIN H, et al. Volatile ester formation in roses. Identification of an acetyl-coenzyme A.Geraniol/citronellol acetyltransferase in developing rose petals[J]. Plant Physiology, 2003, 131(4): 1868-1876.
[65] ELSHARIF S A, BUETTNER A. Structure-odor relationship study on geraniol, nerol, and their synthesized oxygenated derivatives[J]. Journal of Agricultural and Food Chemistry,2018, 66(10): 2324-2333.
[66] DANI K G S, FINESCHI S, MICHELOZZI M. Diversification of petal monoterpene profiles during floral development and senescence in wild roses: Relationships among geraniol content,petal colour, and floral lifespan[J]. Oecologia, 2020.
[67] DUNPHY P J. Location and biosynthesis of monoterpenyl fatty acyl esters in rose petals[J]. Phytochemistry, 2006, 67(11): 1110-1119.
[68] JAYAGANESH R, PUGALENDHI P, MURALI R. Effect of citronellol on NF- κB inflammatory signaling molecules in chemical carcinogen-induced mammary cancer in the rat model[J]. J Biochem Mol Toxicol, 2020, 34(3), e22441.
[69] HIRATA H, OHNISHI T, WATANABE N. Biosynthesis of floral scent 2-phenylethanol in rose flowers[J]. Biosci Biotechnol Biochem, 2016, 80(10): 1865-1873.
[70] TOSHIAKI T, TAKAKI M, MITSURU F, et al. Detection thresholds for phenyl ethyl alcohol using serial dilutions in different solvents[J]. Chemical Senses, 2003, 28(1): 25-32.
[71] YAN H, BAUDINO S, CAISSARD J C, et al. Functional characterization of the eugenol synthase gene (RcEGS1) in rose[J]. Plant Physiology Biochem, 2018, 129: 21-26.
[72] JOARDAR A, MEHER G, BAG B P, et al. Host-guest complexation of eugenol in cyclodextrins for enhancing bioavailability[J]. Journal of Molecular Liquids, 2020, 319.
[73] KHEMIS I, MECHI N, LAMINE A. Investigation of mouse eugenol olfactory receptor activated by eugenol, vanillin and ethyl vanillin: Steric and energetic characterizations[J]. International Journal of Biological Macromolecules, 2020, 163: 2325-2333.
[74] DUDAREVA N, KLEMPIEN A, MUHLEMANN J K, et al.Biosynthesis, function and metabolic engineering of plant volatile organic compounds[J]. New Phytol, 2013, 198(1): 16-32.
[75] ASHITHA G N, SUNNY A C, NISHA R. Effect of pre-harvest and post-harvest hexanal treatments on fruits and vegetables: a review[J]. Agricultural Reviews, 2020, 41(2): 124-131.
[76] ERNSTGÅRD L, DWIVEDI A M, LUNDSTRÖM J N, et al.Measures of odor and lateralization thresholds of acrolein,crotonaldehyde, and hexanal using a novel vapor delivery technique[J]. Plos One, 2017, 12(9): 1-14.
[77] FANG Q, LI Y, LIU B, et al. Cloning and functional characterization of a carotenoid cleavage dioxygenase 2 gene in safranal and crocin biosynthesis from Freesia hybrida[J]. Plant Physiology Biochemistry, 2020, 154: 439-450.
[78] MYKHAILENKO O, KOVALYOV V, GORYACHA O, et al.Biologically active compounds and pharmacological activities of species of the genus crocus: a review[J]. Phytochemistry, 2019,162: 56-89.
[79] SAMARGHANDIAN S, BORJI A, DELKHOSH M B, et al.Safranal treatment improves hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats[J]. Journal of Pharmacy and Pharmaceutical Sciences, 2013, 16(2): 352-362.
[80] SCHWARTZ S H, QIN X, ZEEVAART J D. Characterization of a novel carotenoid cleavage dioxygenase from plants[J]. Journal of Biological Chemistry, 2001, 276(27): 25208-25211.
[81] ANSARI M, EMAMI S. Beta-Ionone and its analogs as promising anticancer agents[J]. European Journal Medicinal Chemistry, 2016, 123: 141-154.
[82] SIMKIN A J, UNDERWOOD B A, AULDRIDGE M, et al.Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of beta-ionone, a fragrance volatile of petunia flowers[J]. Plant Physiol, 2004, 136(3): 3504-3514.
[83] FAN J, ZHANG W, ZHANG D, et al. Flowering Stage and Daytime Affect Scent Emission of Malus ioensis "Prairie Rose"[J]. Molecules, 2019, 24(13).
[84] BONIKOWSKI R, ŚWⅠTAKOWSKA P, KULA J. Synthesis,odour evaluation and antimicrobial activity of some geranyl acetone and nerolidol analogues[J]. Flavour and Fragrance Journal, 2015, 30(3): 238-244.
[85] SHALIT M, KATZIR N, TADMOR Y, et al. Acetyl-CoA:alcohol acetyltransferase activity and aroma formation in ripening melon fruits[J]. Journal of Agricultural and Food Chemistry, 2001, 49(2), 794-799.
[86] APROTOSOAIE A C, HANCIANU M, COSTACHE I, et al.Linalool: A review on a key odorant molecule with valuable biological properties[J]. Flavour and Fragrance Journal, 2014:193-219.
[87] LIAO P C, YANG T S, CHOU J C, et al. Anti-inflammatory activity of neral and geranial isolated from fruits of Litsea cubeba Lour[J]. Journal of Functional Foods, 2015, 19: 248-258.
[88] OSAFUNE Y, TOSHIDA K, HAN J, et al. Characterisation and threshold measurement of aroma compounds contributing to the quality of Honkaku shochu and Awamori[J]. Journal of the Institute of Brewing, 2020, 126(1): 131-135.
[89] BELITZ H D, GROSCH W, SCHIEBERLE P. Food chemistry (4th)[M]. Springer, 2009.
[90] CLARKE S. Essential chemisty for aromatherapy (Second Edition)[M]. Churchill Livingstone, 2008.
[91] HANSSON A, ANDERSSON J, LEUFVEN A, et al. Effect of changes in pH on the release of flavour compounds from a soft drink-related model system[J]. Food Chemistry, 2001: 429-435.
[92] CHEN K, ZHANG M, BHANDARI B, et al. Edible flower essential oils: A review of chemical compositions, bioactivities,safety and applications in food preservation[J]. Food Research International, 2021, 139, 109809.
[93] PICHERSKY E, NOEL J P, DUDAREVA N. Biosynthesis of plant volatiles: nature's diversity and ingenuity[J]. Science,2006, 311(5762), 808-811.
[94] WIDHALM J R, JAINI R, MORGAN J A, et al. Rethinking how volatiles are released from plant cells[J]. Trends Plant Science,2015, 20(9): 545-550.
[95] JORGENSEN U, HANSEN M, CHRISTENSEN L P, et al.Olfactory and quantitative analysis of aroma compounds in elder flower (Sambucus nigra L.) drink processed from five cultivars[J]. Journal of Agricultural and Food Chemistry, 2000: 2376-2383.
[96] FANCELLO F, PETRETTO G, SANNA M L, et al. Isolation and characterization of microorganisms and volatiles associated with Moroccan saffron during different processing treatments[J]. International Journal of Food Microbiology, 2018: 43-49.
[97] QIU L, ZHANG M, BHANDARI B, et al. Effects of infrared freeze drying on volatile profile, FTIR molecular structure profile and nutritional properties of edible rose flower (Rosa rugosa flower) [J]. Journal of the Science of Food and Agriculture, 2020, 100(13): 4791-4800.
[98] KELLEY K M, CAMERON A C, BIERNBAUM J A, et al.Effect of storage temperature on the quality of edible flowers[J]. Postharvest Biology and Technology, 2003: 341-344.
[99] ZHAO L, FAN H, ZHANG M, et al. Edible flowers: Review of flower processing and extraction of bioactive compounds by novel technologies[J]. Food Research International, 2019, 126,108660.
[100] GIL C S, LIM S T, LIM Y J, et al. Volatile content variation in the petals of cut roses during vase life[J]. Scientia Horticulturae, 2020, 261.
[101] WEI L, ZHANG Q, ZHANG, et al. Effects of postharvest chilling and heating treatments on the sensory quality and antioxidant system of daylily flowers[J]. Horticulture, Environment and Biotechnology, 2018: 671-685.
[102] SALVADOR A C, SILVESTRE A J, ROCHA S M. Unveiling elderflowers (Sambucus nigra L.) volatile terpenic and norisoprenoids profile: Effects of different postharvest conditions[J]. Food Chemistry, 2017, 229: 276-285.
[103] PATTARIN L, AURAS R A, BURGESS G J, et al. Preliminary quantification of the permeability, solubility and diffusion coefficients of major aroma compounds present in herbs through various plastic packaging materials[J]. Journal of the Science of Food and Agriculture, 2018: 1545-1553.
[104] WANG C, FANG H, GONG T, et al. Hydrogen gas alleviates postharvest senescence of cut rose ‘Movie star' by antagonizing ethylene[J]. Plant Molecilar Biology, 2020, 102(3): 271-285.
[105] CHRYSARGYRIS A, TZIONIS A, XYLIA P, et al.Physiochemical properties of petunia edible flowers grown under saline conditions and their postharvest performance under modified atmosphere packaging and ethanol application[J].Journal of the Science of Food and Agriculture, 2019, 99(7):3644-3652.
[106] BARANI Y H, ZHANG M, MUJUMDAR A S, et al.Preservation of color and nutrients in anthocyanin-rich edible flowers: progress of new extraction and processing techniques[J]. Journal of Food Processing and Preservation, 2022, 46(9):e16474.
[107] YANG H, LI W, LU B, et al. Effect of different hot air drying process on flavor compounds and Maillard reaction products of Boletus edulis by HS-SPME/GC-MS coupled with multivariate analysis[J]. LWT, 2024, 198, 116055.
[108] CREMER D R, EICHNER K. The reaction kinetics for the formation of Strecker aldehydes in low moisture model systems and in plant powders[J]. Food Chemistry, 2000: 37-43.
[109] SMIT B A, ENGELS W J, SMIT G. Branched chain aldehydes:production and breakdown pathways and relevance for flavour in foods[J]. Appl Microbiol Biotechnol, 2009, 81(6): 987-999.
[110] PRUSINOWSKA R, ŚMⅠGⅠELSKⅠ B. Composition, biological properties and therapeutic effects of lavender (Lavandula angustifolia L). A review[J]. Herba Polonica, 2014, 60(2): 56-66.
[111] SAMAD G, NEDA R. Saffron; an updated review on biological properties with special focus on cardiovascular effects[J]. Biomedicine & Pharmacotherapy, 2019: 21-27.
[112] ANDREW J, SIMKIN B A, MICHELE A, et al. Circadian regulation of the phccd1 carotenoid cleavage dioxygenase controls emission of β-ionone, a fragrance volatile of petunia flowers[J]. Plant Physiology, 2004, 136(3): 3504.
[113] XIAO T, Z, GRANVOGL M. Characterization of the key aroma compounds in two differently dried Toona sinensis (A. Juss.)Roem. by means of the molecular sensory science concept[J]. Journal of Agricultural and Food Chemistry, 2019: 9885-9894.
[114] KWASNICA A, PACHURA N, MASZTALERZ K, et al. Volatile Composition and sensory properties as quality attributes of fresh and dried hemp flowers (Cannabis sativa L.) [J]. Foods, 2020, 9(8).
[115] SHAIMAA E, ASHUTOSH B, ANDREA B. Structure-odor relationships of linalool, linalyl acetate and their corresponding oxygenated derivatives[J]. Frontiers in Chemistry, 2015, 3.
[116] SHI L, GU J, DAN W, et al. Hot air drying of tea flowers: effect of experimental temperatures on drying kinetics, bioactive compounds and quality attributes[J]. International Journal of Food Science & Technology, 2019: 526-535.
备注:英文原文详见P57-70。