餐后高血糖症有很多严重危害并能引起II型糖尿病(餐后糖尿病,postprandial diabetes),据国际糖尿病联盟(International Diabetes Federation,IDF)发布数据显示,2021年全球成年糖尿病患者人数达到5.37亿。据估算,我国糖尿病患病率约为11.2%,糖尿病患者达到1.56亿,已经成为重大的公共卫生问题[1]。IDF和美国糖尿病协会(American Diabetes Association)已经设定了控制餐后血糖的严格目标值[2-3]。食品中碳水化合物尤其是淀粉的消化吸收是引起餐后高血糖症的主要原因。因此,降低食品中碳水化合物的消化速率是预防高血糖症的有效措施。
近年来,全谷物以其对人体健康的重要作用得到了广泛的关注和发展,增加全谷物的摄入可有效降低Ⅱ型糖尿病等非传染性慢性疾病的发病率[4]。全谷物包括糙米、全麦、高粱、燕麦、黑麦等。其中糙米是稻谷垄谷后的产品,再加工脱皮后即可得到大米。糙米属颖果,是由5%~7%糠层、66%~72%淀粉质胚乳和2%~3.5%胚组成的完整果实[5-6]。糙米中含有高达700 mg/kg的酚类物质,其中包括约255~362 mg/kg阿魏酸和70~152 mg/kg p-香豆酸等酚酸类物质[5-6]。糙米中的酚类物质对淀粉的消化有一定的延缓或抑制作用,但其作用机理不清楚。近年来,包括笔者所在团队在内的国内外学者围绕植物多酚与淀粉消化特性等方面开展了相关研究。本文主要综述糙米多酚及其抗氧化活性,阐明糙米多酚对淀粉消化特性的影响及作用机制,以期为淀粉基食品的深度开发提供依据。
1 糙米中的酚类物质组成、含量及结构
多酚是一类广泛存在于植物源食物中,具有多元酚结构的次级代谢产物。根据多酚类化合物化学结构的不同,可分为酚酸类、黄酮类化合物(黄酮、黄酮醇、黄烷酮、黄烷醇、异黄酮以及花青素)以及非黄酮类化合物(芪类和木质素类)等[7]。酚酸是谷物中普遍存在的一种酚类物质,根据其分子结构可分为羟基苯甲酸衍生物和羟基肉桂酸衍生物两类。羟基苯甲酸衍生物主要包括没食子酸、原儿茶酸、对羟基苯甲酸等,羟基肉桂酸类衍生物主要包括肉桂酸、阿魏酸、咖啡酸、对香豆酸等。糙米富含多酚类物质,但其在谷物籽粒中的分布不均匀,主要集中在糠层和糊粉层部分[8]。Ti等选取我国5种广泛种植的籼糙米,对其酚类物质的分布及组成进行探究发现,米糠/胚芽中的总酚和总黄酮含量分别是胚乳中的3.1倍和10.4倍[9]。糙米多酚的含量及组成受稻谷种类、种植区域、麸皮颜色、籽粒大小、提取方式等多种因素的影响[8,10-12]。Ding等研究了来自我国不同种植区的粳稻(Japonica)和籼稻(Indica)的酚酸组成,共鉴定得到12种酚类化合物,其中阿魏酸、没食子酸、原儿茶酸和丁香酸的含量较高;且相较而言,粳稻中的总酚含量显著高于籼稻[13]。笔者所在团队前期研究发现,黑米、粳米和籼米米糠中总酚、总黄酮及总花青素的含量分别分布在163.99~2 351.32 mg GAE/100 g DW,34.06~578.07 mg RE/100 g DW和0.41~1 545.20 mg Cy-3-GE/100 g DW之间[14]。酚类化合物以游离态、共轭态及结合态等不同形式存在于植物体中,与水果、蔬菜中的酚类化合物相比,糙米和其他谷物中60%~85%的酚类化合物多通过酯键、醚间、糖苷键等共价键或者氢键、疏水相互作用与细胞壁中的不溶性结构组分(纤维素、半纤维素、果胶、木质素和结构蛋白等)结合存在[6,15]。结合态多酚需要首先在酯酶或糖苷酶作用下降解断裂后得以释放成为小分子多酚,才能被机体吸收、代谢,因此游离态多酚的生物利用率往往更高,而结合态多酚的吸收利用还需要肠道微生物的协同作用[16-17]。糙米多酚还能够提高细胞内源抗氧化酶系的活性,减少炎症因子的分泌与表达,降低血清中丙二醛(MDA)含量,清除活性氧,最终缓解机体氧化应激水平,降低糖尿病、心血管疾病、癌症等慢性病的发生[18-20]。此外,有色糙米多酚提取物能够显著抑制脂质的积累,通过调控脂肪酸代谢的核心转录因子——过氧化物酶体增殖物激活受体(PPARG)的表达,抑制脂肪细胞的分化[21]。
2 酚类化合物对淀粉消化特性的影响
2.1 淀粉类型及其消化过程
在食品碳水化合物中,淀粉是能量和新陈代谢的主要来源,因而有其独特地位。根据淀粉的消化速率、消化程度及对应的餐后血糖生成值和生理效应,淀粉一般分为快速消化淀粉(rapidly digestible starch, RDS)、缓慢消化淀粉(slowly digestible starch, SDS)和抗性淀粉(resistant starch,RS)[22]。快速消化淀粉迅速消化并且在十二指肠和小肠的近端区域吸收,导致血糖和胰岛素水平快速升高,而抗性淀粉在上消化道不被消化,但在结肠中通过微生物发酵产生有益肠道健康的短链脂肪酸。缓慢消化淀粉在整个小肠中被缓慢消化,使葡萄糖持续缓慢释放且初始血糖值较低,这对人体规律的生理过程和健康是非常重要的[23]。因此,采用酚类物质抑制淀粉的消化速率和消化率,使淀粉中具有较高含量的缓慢消化淀粉和抗性淀粉是目前研究的热点问题。
2.2 酚类物质与消化酶相互作用及对消化酶的抑制作用
近年来的研究表明,利用植物酚类物质是降低淀粉消化速率及人体血糖值的一种有效途径[24-28]。植物酚类化合物是植物次生代谢物的主要类型之一,也是人类膳食中的一类重要成分,在植物体内以游离态和结合态形式存在。由于酚类物质具有的酚羟基结构,使其具有天然抗氧化活性及清除自由基能力, 所以在医药、化工、染料、制革等领域都得到了广泛的应用。
在目前的文献报道中,酚类物质具有降低淀粉消化速率的作用普遍认为是由于酚类物质与淀粉酶、葡(萄)糖苷酶等消化酶发生络合反应,改变酶的构型构象,使酶发生变性或沉淀,抑制酶的活性,从而使淀粉的消化速率降低[29]。笔者所在团队研究发现,黑米米糠游离酚对α-葡萄糖苷酶的抑制率最高可以达到98.2%,其IC50值为8.59±0.03 μg/mL[14]。小麦、大麦和玉米中多种酚酸比单个酚酸对淀粉酶活性抑制作用及淀粉消化率的降低作用效果要好[30]。这些植物酚类物质含有酚羟基团,可能与蛋白分子(酶)的极性基团之间形成了氢键;植物酚类物质及蛋白分子(酶)中含有的疏水基团之间也可能产生疏水相互作用[31]。这两种分子相互作用可能使蛋白分子(酶)的构象发生改变,从而抑制了酶活性。植物酚类物质的结构和类型不同,导致其对淀粉酶、糖化酶等消化酶类活性的不同抑制效率和淀粉消化速率[26]。不同品种的米糠多酚对α-淀粉酶和α-葡萄糖苷酶均具有抑制作用,其中结合态多酚对淀粉酶的抑制率均优于其相应的游离态酚类物质,黑米米糠多酚的作用效果最佳,可能与高含量的花色苷类组成有关[14]。没食子酰基化的儿茶酸比非没食子酰基化的儿茶酸对α-淀粉酶的亲和性要高,且多酚对α-淀粉酶的抑制能力随着没食子酰基的增加显著提升[32-33],而黑茶提取物比绿茶提取物对淀粉消化的抑制率要高[34]。多酚类化合物对消化酶的抑制作用效果与酚羟基的位置和数量有很大关系[35]。考察四种羟基肉桂酸类酚酸物质(肉桂酸、咖啡酸、阿魏酸和3,4-二甲氧基肉桂酸)对α-淀粉酶与α-葡萄糖苷酶的抑制作用发现,4种酚酸对α-淀粉酶活性的抑制效果均优于α-葡萄糖苷酶,其抑制率顺序为咖啡酸>阿魏酸>3,4-二甲氧基肉桂酸>肉桂酸[36]。
2.3 淀粉种类和结构对酚与淀粉相互作用的影响
此外,淀粉的种类和结构会影响多酚与淀粉间的相互作用。当体系中直链淀粉含量越高时,多酚对淀粉消化的抑制作用效果则更明显[37]。多酚与淀粉分子发生复合后,改变淀粉各部分的组成和含量,提高体系中RS和SDS的含量。笔者所在团队研究发现,籼米结合酚提取物的添加使籼米淀粉体系中RS的含量从7.15%增加至24.63%[38]。Ren等的研究发现,相较于儿茶素,阿魏酸提高直链淀粉体系中RS含量的效果更为显著,添加3%浓度剂量的阿魏酸后,体系中RS的含量达到了50%[39]。
2.4 影响酚与淀粉相互作用的其他因素
在酚类物质对淀粉消化性质的影响作用方面,有一些相矛盾的研究结论出现。四种柑橘类黄酮(hesperidin, naringin, neohesperidin,nobiletin)对α-淀粉酶和α-葡(萄)糖苷酶的抑制效率较低,但却有效延缓了淀粉的消化[40]。关于植物酚类物质的单体和聚合物对淀粉消化性的影响作用方面,有研究表明,只有高粱单宁聚合物(每克淀粉中含量为100 mg)在蒸煮过程中提升了普通玉米淀粉、蜡质玉米淀粉和高直链玉米淀粉中抗性淀粉的含量,而其它低分子量的单体多酚提取物则没有任何效果[41];另一研究则表明,在未蒸煮、蒸煮和储藏的天然高粱粉中,高粱单宁浓度较低时(相当于每克粉中0~50 mg儿茶酚),对快速消化淀粉、缓慢消化淀粉和抗性淀粉的含量没有显著的影响作用;然而高粱单宁及儿茶酚分别加入至普通玉米淀粉中时,无论高粱单宁含量高低,均能增加抗性淀粉的含量,而儿茶酚只有在高浓度时才能显著增加抗性淀粉的含量[42]。高粱酚类物质提取物降低了由普通玉米淀粉、高直链玉米淀粉和高粱粉制成的粥中的血糖值和提高了抗性淀粉含量[43],较高含量茶多酚的加入使高直链玉米淀粉中缓慢消化淀粉的量增加[44];而另一研究却表明,茶多酚的存在提高了高直链玉米淀粉的消化率[45]。这些相矛盾的研究结论表明,植物酚类物质对淀粉消化性质的影响作用除了与酚类物质结构和类型有关外,酚类物质的浓度、淀粉的结构和组成、酚类物质-淀粉体系所处的如pH值、高温等的环境条件可能都会影响淀粉的消化性质。可见,酚类物质影响淀粉消化特性的机理需要进行深入探讨。
3 糙米酚类物质对淀粉消化特性的影响机理
糙米具有抗氧化、降血糖、控制血胆固醇及降低心血管疾病风险的作用[46-48],大米淀粉是谷物淀粉颗粒中的最小颗粒,大小范围在3~8 µm,分为高直链淀粉型和蜡质型(高支链淀粉)。因此,我们推断:糙米酚类物质与大米淀粉在不同环境条件下的相互作用,通过形成氢键、疏水基团之间的作用,改变酚类物质及大米淀粉的结构形式,一方面,酚类物质特有基团如酚羟基对消化酶类产生一定的抑制作用,另一方面,大米淀粉结构的改变也使消化酶类对其的作用减小,从而不仅可以有效控制大米淀粉的消化速率及消化率,而且将会改善其食品品质。综上,总结现有研究报道结果,糙米酚类物质降低米淀粉消化速率及血糖生成值主要通过以下两个途径:
(1)糙米酚类物质通过氢键、范德华力、疏水相互作用等分子间作用力与α-淀粉酶和α-葡萄糖苷酶结合,抑制α-淀粉酶和α-葡萄糖苷酶的活性,进而阻止消化酶作用于淀粉分子,从而降低了淀粉的消化速率和消化率,进而降低血糖值(图1)。

图1 糙米酚类物质与消化酶的相互作用机理图
Fig.1 Interaction mechanism diagram between phenolic substances in brown rice and digestive enzymes
(2)糙米酚类物质与淀粉糊化和老化过程中,糙米多酚通过疏水相互作用进入到淀粉分子的疏水双螺旋内部,形成淀粉-多酚V型复合物,减少了消化酶与淀粉分子的结合位点;另一方面多酚与淀粉间通过氢键、范德华力等非共价的相互作用,削弱淀粉的网络凝胶结构,阻碍淀粉的糊化和重结晶,从而使淀粉分子结构改变,更有利于降低淀粉消化速率和消化率,进而降低血糖值(图2)。
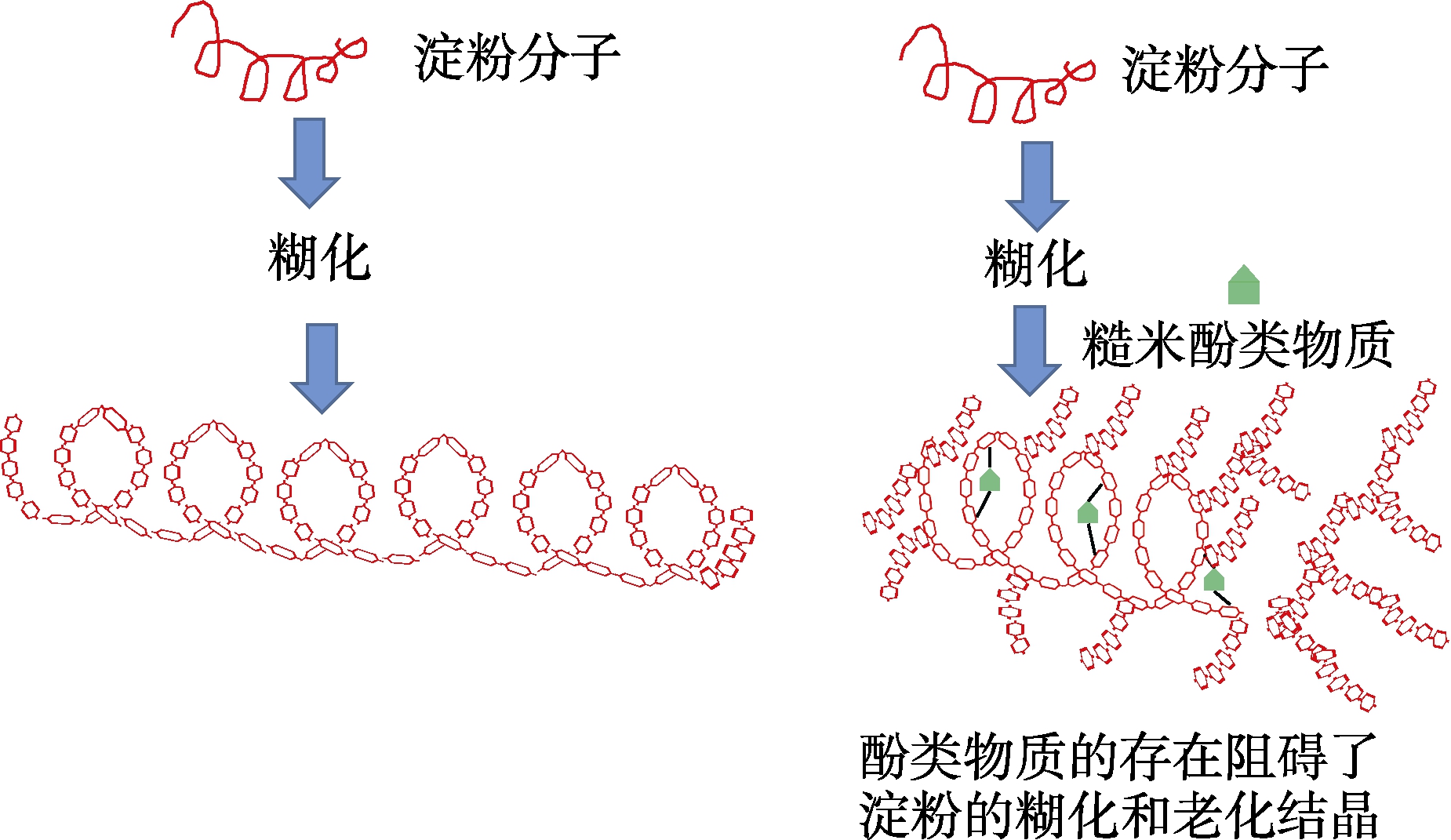
图2 加工过程中糙米酚类物质与淀粉相互作用的机理图
Fig.2 Interaction mechanism diagram between phenolic substances in brown rice and starch during processing
4 小结
我国糙米资源丰富,如何推动糙米和糙米功能食品的开发、利用与消费是食品行业亟需解决的问题。糙米特别是其糠层部分富含多酚类物质,一方面,酚类物质与淀粉消化酶通过相互作用降低酶活,抑制其对淀粉的水解与消化,另一方面,糙米多酚与淀粉颗粒间的复合作用延缓了消化酶对淀粉的识别,从而降低了淀粉的分解速率。本文系统揭示了糙米多酚对淀粉消化特性的影响及其作用机制,为全谷物糙米的深度开发利用提供科学依据。然而,糙米多酚与淀粉间的相互作用还会受到食品基质这个复杂体系的影响。现有研究仍然存在一些不足:(1)糙米多酚-淀粉复合物的结构、存在形式、相互作用关系还有待在分子水平、微观层面进行多尺度的解析;(2)糙米多酚对淀粉消化特性的调节机制及可能参与的代谢通路还需要深入探索,并进一步在不同人群中进行实验与验证,以期为Ⅱ型糖尿病的预防与治疗提供理论支撑;(3)糙米多酚-淀粉复合物在复杂基质中的作用规律还缺乏系统性研究,特别是蛋白、膳食纤维、脂质等组分对其消化特性的影响以及组分间的协同互作机理。
[1] LI Y, TENG D, SHI X, et al.Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study[J].BMJ,2020: m997.
[2] CERIELLO A, COLAGIURI S.International diabetes federation guideline for management of postmeal glucose: A review of recommendations[J].Diabetic Medicine, 2008, 25: 1151-1156.
[3] NATHAN D M, BUSE J B, DAVIDSON M B, et al.Medical management of hyperglycaemia in type 2 diabetes mellitus: A consensus algorithm for the initiation and adjustment of therapy:A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes[J].Diabetologia, 2009, 52: 17-30.
[4] SLAVIN J.Whole grains and human health[J].Nutrition Research Reviews, 2004, 17: 99-110.
[5] KATO T, HORIBATA A.Distribution of γ-oryzanol in the outer layers of brown rice and its variation among cultivars[J].Plant Production Science, 2020, 24(2): 256-265.
[6] ZHOU Z, ROBARDS K, HELLIWELL S, et al.The distribution of phenolic acids in rice[J].Food Chemistry, 2004, 87: 401-406.
[7] ARAVIND S M, WICHIENCHOT S, TSAO R, et al.Role of dietary polyphenols on gut microbiota, their metabolites and health benefits[J].Food Research International, 2021, 142(5):110189.
[8] YU C W, LUO T, XIE T, et al.Classified processing of different rice bran fractions according to their component distributions[J].International Journal of Food Science & Technology, 2022.
[9] TI H, LI Q, ZHANG R, et al.Free and bound phenolic profiles and antioxidant activity of milled fractions of different indica rice varieties cultivated in southern China[J].Food Chemistry,2014, 159(15): 166-174.
[10] TYAGI A, CHEN X, SHABBIR U, et al.Effect of slightly acidic electrolyzed water on amino acid and phenolic profiling of germinated brown rice sprouts and their antioxidant potential[J].LWT-Food Science and Technology, 2022, 157: 113119.
[11] SAHU R, MANDAL S, DAS P, et al.The bioavailability, health advantages, extraction method, and distribution of free and bound phenolics of rice, wheat, and maize: A review[J].Food Chemistry Advances, 2023, 3: 100484.
[12] PANG Y, AHMED S, XU Y, et al.Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice[J].Food Chemistry, 2017, 240: 212.
[13] DING C, LIU Q, LI P, et al.Distribution and quantitative analysis of phenolic compounds in fractions of Japonica and Indica rice[J].Food Chemistry, 2019, 274(15): 384-391.
[14] WU N N, LI H H, TAN B, et al.Free and bound phenolic profiles of the bran from different rice varieties and their antioxidant activity and inhibitory effects on alpha-amylose and alpha- glucosidase[J].Journal of Cereal Science, 2018, 82: 206-212
[15] 张馨文.米糠膳食纤维中结合态多酚的消化代谢特征及其改善糖代谢作用机制[D].武汉: 华中农业大学, 2020.ZHANG X W.Digestive and metabolic characteristics of bound phenolics in rice bran dietary fiber and its mechanism of improved glucose metabolism[D].Wuhan: Huazhong Agricultural University, 2020.
[16] KHAN J, KHAN M Z, MA Y, et al.Overview of the composition of whole grains’ phenolic acids and dietary fibre and their effect on chronic non-communicable diseases[J].International journal of environmental research and public health, 2022, 19(5): 3042.
[17] CĂLINOIU, LAVINIA F, VODNAR D C.Whole grains and phenolic acids: a review on bioactivity, functionality, health benefits and bioavailability[J].Nutrients, 2018, 10(11).
[18] 曾子聪.挤压加工对糙米多酚及其抗氧化性影响的研究[D].南昌: 南昌大学, 2019.ZENG Z C.Study on influence of extrusion processing on phenolic compounds and antioxidant activity of brown rice[D].Nanchang: Nanchang University, 2019.
[19] GHASEMZADEH A, KARBALAII M T, JAAFAR H Z E, et al.Phytochemical constituents, antioxidant activity, and antiproliferative properties of black, red, and brown rice bran[J].Chemistry Central Journal, 2018, 12(1): 17.
[20] SHIN H Y, KIM S M, LEE J H, et al.Solid-state fermentation of black rice bran with Aspergillus awamori and Aspergillus oryzae: Effects on phenolic acid composition and antioxidant activity of bran extracts[J].Food chemistry, 2019, 272: 235-241.
[21] CALLCOTT E T, SANTHAKUMAR A B, STRAPPE P, et al.Polyphenols from Australian-grown pigmented red and purple rice inhibit adipocyte differentiation[J].Journal of Cereal Science, 2018, 81, 140-146.
[22] ENGLYST H N, KINGMAN S M, CUMMINGS J H.Classification and measurement of nutritionally important starch fractions[J].European Journal of Clinical Nutrition, 1992, 46:S30-S50.
[23] ENGLYST K N, ENGLYST H N.Carbohydrate bioavailability[J].British Journal of Nutrition, 2005, 94: 1-11.
[24] FISH B C, THOMPSON L U.Lectin-tannin interactions and their influence on pancreatic amylase activity and starch digestibility[J].Journal of Agricultural and Food Chemistry,1991, 39: 727-731.
[25] XU T, LI X, JI S, et al.Starch modification with phenolics:methods, physicochemical property alteration, and mechanisms of glycaemic control[J].Trends in Food Science & Technology,2021, 111, 12-26.
[26] SUN L J, WARREN F J, GIDLEY M J.Natural products for glycaemic control: Polyphenols as inhibitors of alpha-amylase[J].Trends in Food Science & Technology, 2019, 91, 262-273.
[27] ROCCHETTI G, GIUBERTI G, BUSCONI M, et al.Pigmented sorghum polyphenols as potential inhibitors of starch digestibility:An in vitro study combining starch digestion and untargeted metabolomics[J].Food Chemistry, 2020, 312, 126077.
[28] GIUBERTI G, ROCCHETTI G, LUCINI L.Interactions between phenolic compounds, amylolytic enzymes and starch: an updated overview[J].Current Opinion in Food Science, 2020, 31: 102-113.
[29] DZAH C S, ASANTE-DONYINAH D, LETSYO E, et al.Dietary polyphenols and obesity: a review of polyphenol effects on lipid and glucose metabolism, mitochondrial homeostasis,and starch digestibility and absorption[J].Plant Foods for Human Nutrition.2023, 78(1): 1-12.
[30] KANDIL A, LI J, VASANTHAN T, et al.Phenolic acids in some cereal grains and their inhibitory effect on starch liquefaction and saccharification[J].Journal of Agricultural and Food Chemistry,2012, 60: 8444-8449.
[31] BORDENAVE N, HAMAKER B R, FERRUZZI M G.Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods[J].Food & Function, 2014, 5: 18-34.
[32] MIAO M, JIANG H, JIANG B, et al.Structure elucidation of catechins for modulation of starch digestion[J].LWT - Food Science and Technology, 2014, 57: 188-193.
[33] CAO J, ZHANG Y, HAN L, et al.Number of galloyl moieties and molecular flexibility are both important in alpha-amylase inhibition by galloyl-based polyphenols[J].Food & Function,2020, 11.
[34] GUZAR I, RAGAEE S, SEETHARAMAN K.Mechanism of hydrolysis of native and cooked starches from different botanical sources in the presence of tea extracts[J].Journal of Food Science, 2012, 77: C1192-C1196.
[35] YU M H, ZHU S, LI Y, et al.Role of phenolic acids with different functional groups in the regulation of starch digestion in simulated dietary intake patterns[J].International Journal of Biological Macromolecules, 2023, 235: 123815.
[36] YU M H, ZHU S, HUANG D J, et al, Inhibition of starch digestion by phenolic acids with a cinnamic acid backbone:structural requirements for the inhibition of α-amylase and α-glucosidase[J].Food Chemistry, 2024, 435: 137499.
[37] 任顺成, 陈佳乐, 陶华.多酚对淀粉慢消化作用及其生物利用率研究进展[J].河南工业大学学报(自然科学版), 2022,43(3): 133-140.REN S C, CHEN J L, TAO H.Research progress on the effect of polyphenols on slow digestion of starch and its bioavailability[J].Journal of Henan University of Technology (Natural Science Edition), 2022, 43(3): 133-140.
[38] 李欢欢.糙米多酚对大米淀粉消化特性的影响[D].沈阳: 沈阳师范大学, 2018.LI H H.Effects of polyphenol from brown rice on the digestion characteristics of rice starch[D].Shenyang: Shenyang Normal University, 2018.
[39] REN X, QIN M, ZHANG M, et al.Highland barley polyphenol delayed the in vitro digestibility of starch and amylose by modifying their structural properties[J].Nutrients, 2022, 14(18):3743.
[40] SHEN W, XU Y, LU Y H.Inhibitory effects of citrus flavonoids on starch digestion and antihyperglycemic effects in HepG2 cells[J].Journal of Agricultural and Food Chemistry, 2012, 60:9609- 9619.
[41] BARROS F, AWIKA J M, ROONEY L W.Interaction of tannins and other sorghum phenolic compounds with starch and effects on in vitro starch digestibility[J].Journal of Agricultural and Food Chemistry, 2012, 60: 11609-11617.
[42] MKANDAWIRE N L, KAUFMAN R C, BEAN S R, et al.Effects of sorghum (Sorghum bicolor (L.) Moench) tannins on α-amylase activity and in vitro digestibility of starch in raw and processed flours[J].Journal of Agricultural and Food Chemistry,2013, 61: 4448-4454.
[43] LEMLIOGLU-AUSTIN D, TURNER N D, MCDONOUGH C M, et al.Effects of sorghum [Sorghum bicolor (L.) Moench]crude extracts on starch digestibility, estimated glycemic index(EGI), and resistant starch (RS) contents of porridges[J].Molecules, 2012, 17: 11124-11138.
[44] CHAI Y, WANG M, ZHANG G.Interaction between amylose and tea polyphenols modulates the postprandial glycemic response to high-amylose maize starch[J].Journal of Agricultural and Food Chemistry, 2013, 61: 8608-8615.
[45] LIU J, WANG M, PENG S, et al.Effect of green tea catechins on the postprandial glycemic response to starches differing in amylose content[J].Journal of Agricultural and Food Chemistry,2011, 59: 4582-4588.
[46] SEONG H Y, KIM M.Enhanced protein quality and antioxidant activity of fermented Brown rice with Gryllus bimaculatus[J].LWT-Food Science and Technology, 2021, 150: 111948.
[47] KONGKACHUICHAI R, CHAROENSIRI R, MEEKHRUEROD A, et al.Effect of processing conditions on bioactive compounds and glycemic index of the selected landrace rice variety in pre-diabetes[J].Journal of Cereal Science, 2020, 94: 102994.
[48] WU X, GUO T, LUO F, et al.Brown rice: a missing nutrient-rich health food[J].Food Science and Human Wellness, 2023, 12(5):1458-1470.
备注:本文的彩色图表可从本刊官网(http//lyspkj.ijournal.cn)、中国知网、万方、维普、超星等数据库下载获取。