肥胖已成为全球性的公共卫生问题,会引发一系列慢性代谢疾病,其中II型糖尿病、高血压、高脂血症、心血管疾病等较为常见[1]。肥胖是机体能量摄入超过能量消耗而引起的脂肪过度堆积现象,如过量的食物摄入和较少的体育运动[2]。传统的减肥药主要通过抑制摄食中枢(如利拉鲁肽)或抑制肠道脂肪吸收(如奥利司他)来降低能量摄入,从而达到减肥目的,但这往往会引起一些副作用。因此,通过安全有效的手段增加能量消耗是目前治疗肥胖的新方向。
哺乳动物体内的脂肪组织主要分为白色脂肪组织(white adipose tissue,WAT)和褐色脂肪组织(brown adipose tissue,BAT)。WAT是能量储存的主要场所,而BAT则是消耗能量的脂肪组织[3]。与以单个大脂滴为特征的白色脂肪细胞(white adipocytes,WAC)相比,褐色脂肪细胞(brown adipocytes,BAC)含有大量的线粒体和多房脂滴[4]。BAT通过组织特异性解偶联蛋白 1(uncoupling protein 1,UCP1)介导的非颤抖性产热在能量稳态中起着关键作用。UCP1能将三磷酸腺苷(adenosine triphosphate,ATP)的产生和线粒体呼吸链解偶联,使储存的化学能以热能的形式释放[2]。BAT最早被发现于16世纪,被认为只存在于冬眠的哺乳动物和婴儿体内[5]。直到十多年前,研究者们在成年人体内明确检测到活跃的BAT[6]。近些年,BAT一直被认为是治疗肥胖及相关疾病的潜在靶标[7]。在哺乳动物和婴儿中BAT位于肩胛间区和肾周,在成年人体内存在于颈部和肋间脊髓周围[8]。除了“经典”的WAC和 BAC,另外一种在 WAT中发现的米色脂肪细胞(beige adipocytes,bAC),是一种在特定条件下可诱导的产热脂肪细胞[9]。虽然 BAC和 bAC分布在不同的解剖区域,并有不同的发育起源,但它们表现出许多相似的形态和代谢特征,包括大量的线粒体、多房脂滴和 UCP1表达等[10](图1)。
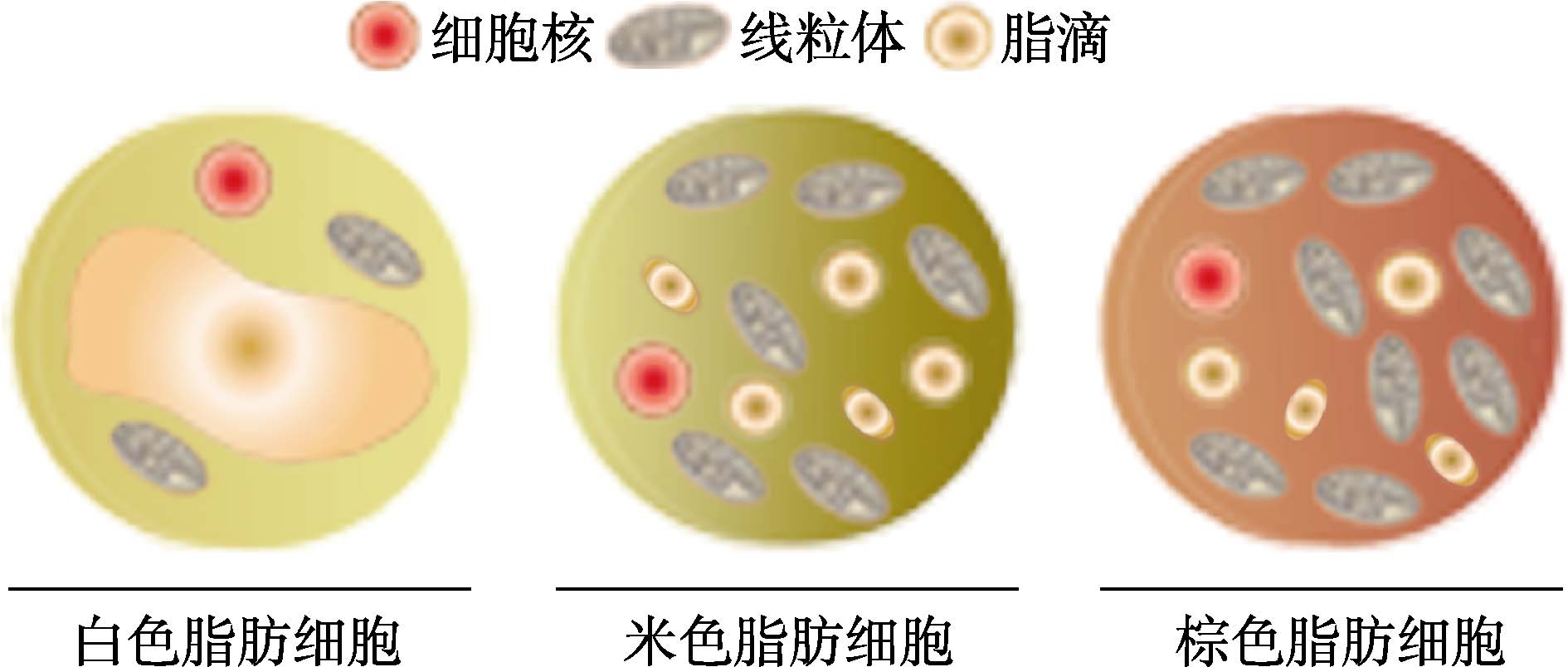
图1 脂肪细胞的三种类型
Fig.1 Three types of adipocytes
BAC和 bAC为肥胖等相关疾病的治疗提供了潜在的靶标。现如今已经发现或合成了许多BAC和 bAC的外源性激活物,如米拉贝隆、西布曲明、氟西汀和罗格列酮等药理因子,以及辣椒素、白藜芦醇、姜黄素、类胡萝卜素、花青素等营养因子。然而,一些药理因子对人体有不良影响,如引发心血管疾病,因此食物中的营养因子对于BAC和bAC是更加健康安全的调节剂[11-12]。
谷物是满足人类营养需求的日常主食资源,精制谷物是谷物产品的主要原料。然而,由于谷物的外层和胚芽被碾磨加工去除,精制谷物缺乏膳食纤维(dietary fiber,DF)、酚类化合物和其他生物活性物质,导致许多代谢综合征的风险增加[13]。全谷物比精制谷物更健康,并且已经在许多国家受到密切关注[14]。美国谷物化学家协会将全谷物食品定义为:完整、碾碎、破碎或压片的颖果,基本的组成包括淀粉质胚乳、胚芽与麸皮,各组成部分的相对比例与完整颖果一样[15]。很多研究表明,全谷物中多酚、类胡萝卜素、植物甾醇、矿物质和维生素[16-17]等生物活性化合物的含量较高,有助于改善代谢综合征,包括肥胖、高血压、高脂血症和高血糖症。
本文简要介绍了产热脂肪细胞的基本功能特征和主要调节因素,总结了全谷物功能成分通过调节产热脂肪细胞功能影响能量代谢的研究进展,希望能够为全谷物调节肥胖和全谷物功能食品开发提供更丰富的依据。
1 产热脂肪细胞
1.1 褐色和米色脂肪细胞的发育
BAC和 bAC在发育起源上是不同的。BAC发育于胚胎期,而 bAC的发育则是在出生后。BAC具有相对稳定的质量水平,并且通过成年后的稳态机制维持着产热表型,而bAC会因寒冷或其他刺激而大量募集,具有可塑性的产热表型,且很少在热中性时检测到[10]。
BAC起源于祖细胞,其特征在于同源异型盒基因 1(engrailed 1,En1)、肌源性因子 5(myogenic factor 5,Myf5)和配对盒蛋白 7(paired box 7,Pax7)的表达,这些因子在骨骼肌的发育中也起关键作用[8-9]。在 BAC胚胎发育过程中,这些祖细胞首先发育为褐色前脂肪细胞,然后再分化为成熟的BAC,许多信号通路和信号因子参与到BAC的发育调节中[4]。
出生后的bAC由WAT中的非肌源性祖细胞响应寒冷或其他刺激发育形成,然而它的胚胎起源比较复杂,尚未完全阐明[9,12,18]。现有报道认为bAC有三种主要的发育机制[10]。第一种是从头脂肪合成机制,某些脂肪血管壁细胞或前脂肪细胞可以发育成bAC;第二种机制是表达脂联素(一种成熟脂肪细胞的分子标记物)的 WAC在寒冷或β肾上腺素激动剂的刺激下转化为bAC;最后一种可能的机制是,在寒冷或β肾上腺素刺激下休眠的bAC可以被快速激活,成为活跃的米色脂肪细胞。然而后面两种机制很难被区分。
1.2 褐色和米色脂肪细胞的产热机制
脂肪细胞的产热能力对能量代谢具有重要意义。UCP1被认为是脂肪细胞产热能力的标志[19]。但最近的研究表明还有其他不依赖UCP1的机制可调节bAC的产热作用[20]。
UCP1位于线粒体内膜上,可以消除内膜两侧的质子浓度差,使氧化磷酸化过程减慢,阻碍ATP产生,使电子传递链和氧化磷酸化解偶联,导致氧化磷酸化进入空转,从而产热[21]。在静息态,由于胞质侧的嘌呤核苷酸和UCP1相结合,UCP1的活性受到抑制。在寒冷或过量摄食的刺激下,交感神经分泌去甲肾上腺素,去甲肾上腺素作用于脂肪细胞质膜上的β肾上腺素受体,导致环磷酸腺苷(cyclic adenosine monophosphate,cAMP)依赖的蛋白激酶(protein kinase,PKA)的激活和激素敏感性脂肪酶(hormone-sensitive lipase,HSL)磷酸化水平的提高。由cAMP诱导的脂解作用产生的游离脂肪酸(free fatty acid,FFA)或是脂肪细胞从循环中吸收获得的 FFA都会与UCP1结合以激活脂肪细胞的产热活性[10,22]。此外,UCP1依赖性产热还可以通过许多转录因子来增强,包括甲状腺激素、过氧化物酶体增殖激活受体(peroxisome proliferator activated receptor,PPAR)、C/EBP家族蛋白和cAMP反应元件结合蛋白(cAMP response binding protein,CREB)[21]。
最近的研究结果表明[23],bAC中UCP1不依赖的产热机制是由多种通路介导的,包括ATP依赖的钙离子循环[24]、肌酸无效循环、脂质循环、3-磷酸甘油穿梭和冷诱导的N-酰基氨基酸解偶联剂等[25]。
2 全谷物功能成分对产热脂肪细胞的影响
2.1 酚酸
全谷物中存在多种可以有效提高产热脂肪细胞活性的酚酸,如香草酸、没食子酸、绿原酸。香草酸(4-羟基-3-甲氧基苯甲酸)是二羟基苯甲酸的衍生物,是香草醛和膳食酚的氧化形式[26],存在于有色大米[27]、全麦和黑麦麦芽中[28]。Jung等[26]研究发现,香草酸促进成熟和原代培养的BAC中线粒体和产热相关因子的表达,而降低白色前脂肪细胞(3T3L1)中成脂标记物的表达。0.5%(w/w)香草酸干预14周促进高果糖高脂饮食(high-fructose and high-fat diet,HFFD)小鼠的BAT和腹股沟白色脂肪(inguinal white adipose tissue,iWAT)中产热和线粒体合成基因的表达,表明香草酸可通过激活BAT产热和促进iWAT褐变来预防肥胖[30]。没食子酸(3,4,5-三羟基苯甲酸)存在于在水稻,玉米和小麦中[27,31]。研究表明没食子酸可激活腺苷单磷酸激活蛋白激酶(adenosine monophosphate-activated protein kinase,AMPK),并增加C57BL/6小鼠BAT中UCP1以及与能量消耗相关基因的表达[32]。此外,没食子酸可以增加雄性瑞士小鼠BAT中Sirt1和PPARγ共激活因子1α(peroxisome proliferator activated receptor-γ coactivator 1α,PGC1α)的mRNA表达,而Sirt1是在没食子酸处理下BAT产热的主要靶标[33]。绿原酸(5-O-咖啡酰奎宁酸)是羟基肉桂酸的衍生物,存在于小麦、玉米和薏苡中[30,33-34]。研究发现绿原酸可通过上调产热相关蛋白、增加线粒体的质子泄漏并减少 ATP的产生来增强 BAC中UCP1依赖性的产热作用,但绿原酸并不能刺激BAC分化[35]。以上研究表明酚酸可通过激活AMPK或Sirt1增加BAC和bAC的产热作用并改善线粒体功能。目前,不同类型酚酸的具体作用机理及效果对比还没有更深入的研究,且相关人体临床试验也需要进一步开展。
2.2 花色苷
矢车菊素-3-O-葡萄糖苷(cyanidin-3-glucoside,C3G)是有色谷物中的主要花色苷之一,如黑米、紫米和一些特定的玉米品种。不同谷物中的C3G含量不同,粗黑米糠,甜玉米和蓝玉米杂种的C3G含量分别约为104.20、4.97和2.25 mg/100 g[36-39]。C3G能够显著影响产热脂肪细胞的能量代谢和产热。You等[40]的研究表明,C3G在褐色脂肪形成过程中增加了UCP1、PRDM16和PGC1α等产热相关基因在小鼠C3H10T1/2细胞(小鼠间充质干细胞)中的表达。You等[41]还发现 C3G能够提高BAT-cMyc细胞中线粒体生物合成相关基因的表达,包括线粒体转录因子 A(mitochondrial transcription factor A,TFAM)和核呼吸因子 2(nuclear respiratory factor 2,NRF2)。C3G 以剂量依赖的方式(50和100 μmol/L)增加胞内cAMP水平从而诱导3T3L1脂肪细胞中bAC的形成[42]。研究表明C3G通过激活Sirt1-PGC1α-TFAM信号通路促进db/db小鼠(糖尿病小鼠)BAT中线粒体的生物合成和皮下白色脂肪组织(subcutaneous white adipose tissue,sWAT)中米色脂肪的形成[43]。C3G还可以通过增强BAT产热和iWAT褐变来减轻 HFFD诱导的C57BL/6J小鼠肥胖症状[44]。因此,C3G可以通过增强脂肪产热来消耗能量,有效预防肥胖。
此外,有研究者[45]指出,部分C3G可以与血液循环中血红蛋白上的一组等效位点结合,而游离的C3G与结合的C3G处于平衡状态,当血浆中游离C3G的含量与血红蛋白的浓度比大于0.1时,游离的C3G可从血液循环转运至靶细胞。除C3G外,在稻米、小麦和黑麦中存在的矢车菊素-3-芸苔苷(cyanidin-3-rutinoside,C3R)[46]可通过激活丝氨酸-苏氨酸激酶(serine-threonine kinase,AKT)和胞外信号相关激酶-丝裂原活化蛋白激酶(extracellular signal-related kinase-mitogen-activated protein kinase,ERK-MAPK)信号通路来激活BAT产热[41]。但是,尚未发现全谷物中其他花色苷对产热脂肪细胞调节作用的研究。
2.3 膳食纤维
全谷物中含有大量DF,可以降低许多慢性疾病的发生率[47]。在高脂饮食(high fat diet,HFD)小鼠中,小麦和燕麦 DF通过激活 UCP1促进WAT褐变,并通过cAMP-PKA-HSL途径增强脂肪细胞的脂解作用。小麦和燕麦 DF干预降低了HFD小鼠的体重,并且DF增加了与WAT褐变和脂解相关蛋白的表达。燕麦DF喂养的HFD小鼠WAT中 PKA、PGC1α和 UCP1的表达高于小麦DF喂养的HFD小鼠,而小麦DF比燕麦DF更有效地增加 BAT中 PKA、UCP1、FGF21、脂肪三酰甘油脂酶和 β3-肾上腺素受体的表达[48]。抗性淀粉是全谷物中的另一种 DF[49],研究表明高抗性淀粉饮食可以增加饮食诱导肥胖大鼠的BAT中UCP1的表达,并有效控制肥胖[50]。
β-葡聚糖是全谷物中主要的膳食纤维之一。已有研究发现[51],燕麦β-葡聚糖对小鼠肝脏和脾脏具有显著的保护作用,并且还能降低由高脂饮食引起的血清总胆固醇、甘油三酯和低密度脂蛋白胆固醇的升高,燕麦β-葡聚糖显著上调PPARα基因的表达,下调脂肪和胆固醇合成相关基因的表达,表明燕麦β-葡聚糖可以通过促进能量消耗及减少脂肪和胆固醇合成来控制体重,因此,可以推测,β-葡聚糖或许在产热脂肪细胞的功能调控中也发挥作用,还需要进一步验证。
2.4 类胡萝卜素
玉米黄质和叶黄素是两种脂溶性抗氧化剂,属于类胡萝卜素的叶黄素类[52]。玉米黄质是叶黄素的立体异构体,它们通常在自然资源中共存[53]。人体无法合成玉米黄质和叶黄素,必须通过饮食摄取[54],尤其是玉米黄质[55]。叶黄素可以通过诱导UCP1的表达来增加BAC的产热作用,但会轻微影响BAC的增殖活性[56]。Liu等[57]研究了不同浓度(5、10、15 μM)玉米黄质对3T3L1前脂肪细胞中BAC形成和线粒体生物发生的影响,发现玉米黄质可以促进褐色/米色脂肪标记物的表达和线粒体生物发生,并且是通过激活AMPKα1实现的。目前,有关玉米黄质或叶黄素对产热脂肪细胞活性影响的研究还非常少,尤其是体内实验。
2.5 植醇及其衍生物植酸
植醇(3,7,11,15-四甲基十六烷基-2-烯-1-醇)是叶绿素的组成部分,属于二萜类精油,存在于粮食作物的绿叶[58]和荞麦的可食用部分[59]。植酸(3,7,11,15-四甲基十六酸)是植醇的衍生物,是一种甲基支链脂肪酸,存在于小麦、大麦和水稻等谷物中。植酸是在乙醇脱氢酶存在的情况下由植醇降解产生的,植醇首先转化为醛,然后醛转化并还原生成植酸。在人体内游离植醇不能转化为植酸,植酸也不能大量积累[60-61]。
以 0.2%或 0.5%(w/w)植醇干预高脂饮食KK-Ay小鼠(一种非胰岛素依赖型糖尿病模型)5周后,BAC中的植酸水平呈剂量依赖性升高,激活PPARα并减轻肥胖引起的代谢性疾病[62]。植醇还可以通过激活AMPKα诱导小鼠iWAT褐变,并促进 3T3L1细胞分化过程中 bAC的形成[63]。植酸可促进小鼠BAC分化和UCP1基因的转录,从而激活BAT的产热活性[64]。而且,植酸可通过激活PPARα促进3T3L1前脂肪细胞的褐化,但不能促进C3H10T1/2细胞向BAC细胞系分化[65]。因此,植醇和植酸能够提高产热脂肪细胞的活性。
植醇和植酸主要通过激活PPARα或AMPKα促进BAC和bAC分化来控制肥胖和相关疾病。此外,有研究推测植醇对BAC分化的促进作用可能部分由植酸引起[63],但植醇和植酸在其中的具体作用机制还需进一步研究。
2.6 芦丁
芦丁属于一种常见的膳食类黄酮[66]。在谷物中,芦丁主要存在于荞麦,在苦荞中的含量远高于普通荞麦[67]。Gao等[68]研究发现,芦丁干预HFD小鼠8周后可显著上调BAC中能量消耗相关基因的表达,降低炎症因子的表达。100 mg/kg芦丁干预3周可增强脱氢表雄酮诱导的多囊卵巢综合征大鼠的BAC活性,从而促进产热,并改善全身胰岛素敏感性[69]。据报道[70],芦丁还可以通过激活 Sirt1-PGC1α-TFAM 信号通路促进 sWAT中bAC的形成及提高BAC的活性。因此,芦丁对产热脂肪细胞的调节起着重要作用,有利于增加能量消耗并降低炎症。
2.7 二十八烷醇
二十八烷醇是一种高分子量的天然脂肪醇,广泛存在于米糠和小麦胚芽中[71]。SHARMA等[72]使用60 mg/kg二十八烷醇对HFD和正常饮食小鼠灌胃给药4周后,两组小鼠的iWAT和BAT中产热相关基因的表达均明显增强,表明二十八烷醇可以通过提高产热脂肪细胞的活性来维持体重并改善饮食诱导的肥胖。然而,二十八烷醇对iWAT和BAT适应性产热作用的确切分子机制有待阐明。
3 展望
BAC和 bAC由于其显著的产热和能量消耗能力成为控制体重和能量代谢的有效靶标。调节BAC和 bAC的信号通路和相关因子多种多样,全谷物中的多种功能性成分可通过 Sirtl-PGC1α-TFAM、AKT/ERK MAPK、cAMP-PKA-HSL及AMPK信号通路调节BAC和bAC的活性。除了产热作用,全谷物膳食还可以通过调节产热脂肪活性改善机体胰岛素敏感性和脂质代谢,因此,增加全谷物的摄入对人体健康是非常有益的。
后续关于全谷物功能成分调节产热脂肪代谢的研究可以从以下几个方面开展:(1)对全谷物功能成分在体内的代谢产物进行鉴定,这些代谢物可能是影响产热脂肪更直接的物质;(2)开展人体实验,明确适合临床试验的相对更有效的功能成分,并进行相关应用性研究;(3)根据不同性别、年龄、肥胖诱因制定含有不同功能成分及配比的全谷物膳食。这将有助于更细致地理解全谷物功能成分对产热脂肪活性的调控机理,进而为全谷物功能食品的进一步开发提供更丰富的依据。
[1]ROHDE K,KELLER M,LA COUR POULSEN L,et al.Genetics and epigenetics in obesity[J].Metabolism Clinical and Experimental,2019,92:37-50.
[2]MARLATT K L,RAVUSSIN E.Brown adipose tissue:an update on recent findings[J].Current Obesity Reports,2017,6:389-396.
[3]CHEN Y,PAN R,PFEIFER A.Regulation of brown and beige fat by microRNAs[J].Pharmacology & Therapeutics,2017,170:1-7.
[4]WANG W,SEALE P.Control of brown and beige fat development[J].Nature Reviews Molecular Cell Biology,2016,17:691-702.
[5]CANNON B,NEDERGAARD J.Brown adipose tissue:function and physiological significance[J].Physiological Reviews,2004,84:277-359.
[6]CYPESS A M,LEHMAN S,WILLIAMS G,et al.Identification and importance of brown adipose tissue in adult humans[J].New England Journal of Medicine,2009,360:1509-1517.
[7]BETZ M J,ENERBACK S.Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease[J].Nature Reviews Endocrinology,2018,14:77-87.
[8]IKEDA K,MARETICH P,KAJIMURA S.The common and distinct features of brown and beige adipocytes[J].Trends in Endocrinology and Metabolism,2018,29:191-200.
[9]WANG S,PAN M H,HUNG W L,et al.From white to beige adipocytes:therapeutic potential of dietary molecules against obesity and their molecular mechanisms[J].Food & Function,2019,10:1263-1279.
[10]RUI L.Brown and beige adipose tissues in health and disease[J].Comprehensive Physiology,2017,7:1281-1306.
[11]MONTANARI T,POSCIC N,COLITTI M.Factors involved in white-to-brown adipose tissue conversion and in thermogenesis:a review[J].Obesity Reviews,2017,18:495-513.
[12]OKLA M,KIM J,KOEHLER K,et al.Dietary factors promoting brown and beige fat development and thermogenesis[J].Advances in Nutrition,2017,8:473-483.
[13]BENÍTEZ V,ESTEBAN R M,MONIZ E,et al.Breads fortified with wholegrain cereals and seeds as source of antioxidant dietary fibre and other bioactive compounds[J].Journal of Cereal Science,2018,82:113-120.
[14]NUGENT A P,THIELECKE F.Wholegrains and health:Many benefits but do contaminants pose any risk[J].Nutrition Bulletin,2019,44:107-115.
[15]谭斌,谭洪卓,刘明,等.粮食(全谷物)的营养与健康[J].中国粮油学报,2010,25(4):100-107.TAN B,TAN H Z,LIU M,et al.The grain the wholegrain:nutrition and health benefits[J].Journal of the Chinese Cereals and Oils Association,2010,25(4):100-107.
[16]PARK H Y,LEE K W,CHOI H D.Rice bran constituents:immunomodulatory and therapeutic activities[J].Food & Function,2017,8:935-943.
[17]GONG L,CAO W,CHI H,et al.Whole cereal grains and potential health effects:Involvement of the gut microbiota[J].Food Researech International,2018,103:84-102.
[18]WHITE J D,DEWAL R S,STANFORD K I.The beneficial effects of brown adipose tissue transplantation[J].Molecular Aspects of Medicine,2019,68:74-81.
[19]LUIJTEN I H N,CANNON B,NEDERGAARD J.Glucocorticoids and brown adipose tissue:Do glucocorticoids really inhibit thermogenesis[J].Molecular Aspects of Medicine,2019,68:42-59.
[20]GAUDRY M J,KEUPER M,JASTROCH M.Molecular evolution of thermogenic uncoupling protein 1 and implications for medical intervention of human disease[J].Molecular Aspects of Medicine,2019,68:6-17.
[21]SEBAA R,JOHNSON J,PILEGGI C,et al.SIRT3 controls brown fat thermogenesis by deacetylation regulation of pathways upstream of UCP1[J].Molecular Metabolism,2019,25:35-49.
[22]KAJIMURA S,SAITO M.A new era in brown adipose tissue biology:molecular control of brown fat development and energy homeostasis[J].Annual Review of Physiology,2014,76:225-249.
[23]CHOUCHANI E T,KAZAK L,SPIEGELMAN B M.New advances in adaptive thermogenesis:UCP1 and beyond[J].Cell Metabolism,2019,29:27-37.
[24]IKEDA K,KANG Q,YONESHIRO T,et al.UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis[J].Nature Medicine,2017,23:1454-1465.
[25]CHANG S H,SONG N J,CHOI J H,et al.Mechanisms underlying UCP1 dependent and independent adipocyte thermogenesis[J].Obesity Reviews,2019,20:241-251.
[26]JUNG Y,PARK J,KIM H L,et al.Vanillic acid attenuates obesity via activation of the AMPK pathway and thermogenic factors in vivo and in vitro[J].FASEB Journal,2018,32:1388-1402.
[27]PANG Y,AHMED S,XU Y,et al.Bound phenolic compounds and antioxidant properties of whole grain and bran of white,red and black rice[J].Food Chemistry,2018,240:212-221.
[28]RIPARI V,BAI Y,GANZLE M G.Metabolism of phenolic acids in whole wheat and rye malt sourdoughs[J].Food Microbiology,2019,77:43-51.
[29]HAN X,GUO J,YOU Y,et al.Vanillic acid activates thermogenesis in brown and white adipose tissue[J].Food & Function,2018,9:4366-4375.
[30]NIKOLIĆ N,MITROVIĆ J,KARABEGOVIĆ I,et al.A comparison between wheat and different kinds of corn flour based on minerals,free phenolic acid composition and antioxidant activity[J].Quality Assurance and Safety of Crops & Foods,2019,11:341-349.
[31]DOAN K V,KO C M,KINYUA A W,et al.Gallic acid regulates body weight and glucose homeostasis through AMPK activation[J].Endocrinology,2015,156:157-168.
[32]PARAISO A F,SOUSA J N,ANDRADE J M O,et al.Oral gallic acid improves metabolic profile by modulating SIRT1 expression in obese mice brown adipose tissue:A molecular and bioinformatic approach[J].Life Sciences,2019,237:116914.
[33]ZHAO M,YANG Q,LIN L,et al.Intracellular antioxidant activities of selected cereal phenolic extracts and mechanisms underlying the protective effects of adlay phenolic extracts on H2O2-induced oxidative stress in human erythrocytes[J].Journal of Functional Foods,2017,31:160-171.
[34]LIMWACHIRANON J,HUANG H,LI L,et al.Enhancing stability and bioaccessibility of chlorogenic acid using complexation with amylopectin:A comprehensive evaluation of complex formation,properties,and characteristics[J].Food Chemistry,2020,311:125879.
[35]HAN X,ZHANG Y,GUO J,et al.Chlorogenic acid stimulates the thermogenesis of brown adipocytes by promoting the uptake of glucose and thefunction of mitochondria[J].Journal of Food Science,2019,84:3815-3824.
[36]LOYPIMAI P,MOONGNGARM A,CHOTTANOM P.Thermal and pH degradation kinetics of anthocyanins in natural food colorant prepared from black rice bran[J].Journal of Food Science and Technology-Mysore,2016,53:461-470.
[37]LOYPIMAI P,MOONGNGARM A,CHOTTANOM P.Extraction solvents affecting phytochemicals in food colorant prepared from purple glutinous rice bran[J].Applied Biological Chemistry,2017,60:181-189.
[38]DAS A K,SINGH V.Antioxidative free and bound phenolic constituents in botanical fractions of Indian specialty maize (Zea mays L.) genotypes[J].Food Chemistry,2016,201:298-306.
[39]OLIVAS-AGUIRRE F J,RODRIGO-GARCIA J,MARTINEZRUIZ N D,et al.Cyanidin-3-O-glucoside:Physical-chemistry,foodomics and health effects[J].Molecules,2016,21:1264.
[40]YOU Y,YUAN X,LEE H J,et al.Mulberry and mulberry wine extract increase the number of mitochondria during brown adipogenesis[J].Food & Function,2015,6:401-408.
[41]YOU Y,LIANG C,HAN X,et al.Mulberry anthocyanins,cyanidin 3-glucoside and cyanidin 3-rutinoside,increase the quantity of mitochondria during brown adipogenesis[J].Journal of Functional Foods,2017,36:348-356.
[42]MATSUKAWA T,VILLAREAL M O,MOTOJIMA H,et al.Increasing cAMP levels of preadipocytes by cyanidin-3-glucoside treatment induces the formation of beige phenotypes in 3T3-L1 adipocytes[J].Journal of Nutritional Biochemistry,2017,40:77-85.
[43]YOU Y,YUAN X,LIU X,et al.Cyanidin-3-glucoside increases whole body energy metabolism by upregulating brown adipose tissue mitochondrial function[J].Molecular Nutrition & Food Research,2017,61(11):1700261.
[44]YOU Y,HAN X,GUO J,et al.Cyanidin-3-glucoside attenuates high-fat and high-fructose diet-induced obesity by promoting the thermogenic capacity of brown adipose tissue[J].Journal of Functional Foods,2018,41:62-71.
[45]TANG L,LI S,BI H,et al.Interaction of cyanidin-3-O-glucoside with three proteins[J].Food Chemistry,2016,196:550-559.
[46]ZHU F.Anthocyanins in cereals:Composition and health effects[J].Food Research International,2018,109:232-249.
[47]ROBINSON E,CHAMBERS L.The challenge of increasing wholegrain intake in the UK[J].Nutrition Bulletin,2018,43:135-146.
[48]HAN S F,JIAO J,ZHANG W,et al.Lipolysis and thermogenesis in adipose tissues as new potential mechanisms for metabolic benefits of dietary fiber[J].Nutrition,2017,33:118-124.
[49]BIRD A R,REGINA A.High amylose wheat:A platform for delivering human health benefits[J].Journal of Cereal Science,2018,82:99-105.
[50]AZIZ A A,KENNEY L S,GOULET B,et al.Dietary starch type affects body weight and glycemic control in freely fed but not energy-restricted obese rats[J].The Journal of Nutrition,2009,139:1881-1889.
[51]刘博.燕麦葡聚糖抗炎与降脂功能的评估及其分子机理研究[D].长沙:中南林业科技大学,2017.LIU B.The anti-inflammation and anti-hyperlipidemia effects and the potential molecular mechanism of oat-beta-glucan[D].Changsha:Central South University of Forestry & Technology,2017.
[52]EISENHAUER B,NATOLI S,LIEW G,et al.Lutein and zeaxanthin-food sources,bioavailability and dietary variety in age-related macular degeneration protection[J].Nutrients,2017,9:120-133.
[53]NWACHUKWU I D,UDENIGWE C C,ALUKO R E.Lutein and zeaxanthin:Production technology,bioavailability,mechanisms of action,visual function,and health claim status[J].Trends in Food Science & Technology,2016,49:74-84.
[54]JIA Y P,SUN L,YU H S,et al.The pharmacological effects of lutein and zeaxanthin on visual disorders and cognition diseases[J].Molecules,2017,22:610.
[55]GIORDANO E,QUADRO L.Lutein,zeaxanthin and mammalian development:Metabolism,functions and implications for health[J].Archives of Biochemistry and Biophysics,2018,647:33-40.
[56]SERRA F,BONET M L,PUIGSERVER P,et al.Stimulation of uncoupling protein 1 expression in brown adipocytes by naturally occurring carotenoids[J].International Journal of Obesity,1999,23:650-655.
[57]LIU M,ZHENG M,CAI D,et al.Zeaxanthin promotes mitochondrial biogenesis and adipocyte browning via AMPKα1 activation[J].Food & Function,2019,10:2221-2233.
[58]PRABHA S P,KARTHIK C,CHANDRIKA S H.Phytol - A biosurfactant from the aquatic weed Hydrilla verticillata[J].Biocatalysis and Agricultural Biotechnology,2019,17:736-742.
[59]RCSTAJ.Standard tables of food composition in Janpan.(5th ed.).Tokyo:PBMF,2000.
[60]ROCA-SAAVEDRA P,MARINO-LORENZO P,MIRANDA J M,et al.Phytanic acid consumption and human health,risks,benefits and future trends:A review[J].Food Chemistry,2017,221:237-247.
[61]DAI F,WANG J,ZHANG S,et al.Genotypic and environmental variation in phytic acid content and its relation to protein content and malt quality in barley[J].Food Chemistry,2007,105:606-611.
[62]AN J Y,JHENG H F,NAGAI H,et al.A phytol-enriched diet activates PPAR-α in the liver and brown adipose tissue to ameliorate obesity-induced metabolic abnormalities[J].Molecular Nutrition & Food Research,2018,62:e1700688.
[63]ZHANG F,AI W,HU X,et al.Phytol stimulates the browning of white adipocytes through the activation of AMP-activated protein kinase (AMPK)α in mice fed high-fat diet[J].Food & Function,2018,9:2043-2050.
[64]SCHLUTER A,GIRALT M,IGLESIAS R,et al.Phytanic acid,but not pristanic acid,mediates the positive effects of phytol derivatives on brown adipocyte differentiation[J].FEBS Letters,2002,517:83-86.
[65]WANG H,MAO X,DU M.Phytanic acid activates PPARα to promote beige adipogenic differentiation of preadipocytes[J].Journal of Nutritional Biochemistry,2019,67:201-211.
[66]GULLÓN B,LÚ-CHAU T A,MOREIRA M T,et al.Rutin:A review on extraction,identification and purification methods,biological activities and approaches to enhance its bioavailability[J].Trends in Food Science & Technology,2017,67:220-235.
[67]OH M,OH I,JEONG S,et al.Optical,rheological,thermal,and microstructural elucidation of rutin enrichment in Tartary buckwheat flour by hydrothermal treatments[J].Food Chemistry,2019,300:125193.
[68]GAO M,MA Y,LIU D.Rutin suppresses palmitic acids-triggered inflammation in macrophages and blocks high fat diet-induced obesity and fatty liver in mice[J].Pharmaceutical Research,2013,30:2940-2950.
[69]HU T,YUAN X,YE R,et al.Brown adipose tissue activation by rutin ameliorates polycystic ovary syndrome in rat[J].Journal of Nutritional Biochemistry,2017,47:21-28.
[70]YUAN X,WEI G,YOU Y,et al.Rutin ameliorates obesity through brown fat activation[J].FASEB Journal,2017,31:333-345.
[71]LI D,LI X,WU G,et al.The characterization and stability of the soy protein isolate/1-Octacosanol nanocomplex[J].Food Chemistry,2019,297:124766.
[72]SHARMA R,MATSUZAKA T,KAUSHIK M K,et al.Octacosanol and policosanol prevent high-fat diet-induced obesity and metabolic disorders by activating brown adipose tissue and improving liver metabolism[J].Scientific Reports,2019,9:5169.
备注:本文的彩色图表可从本刊官网(http://lyspkj.ijournal.cn)、中国知网、万方、维普、超星等数据库下载获取。